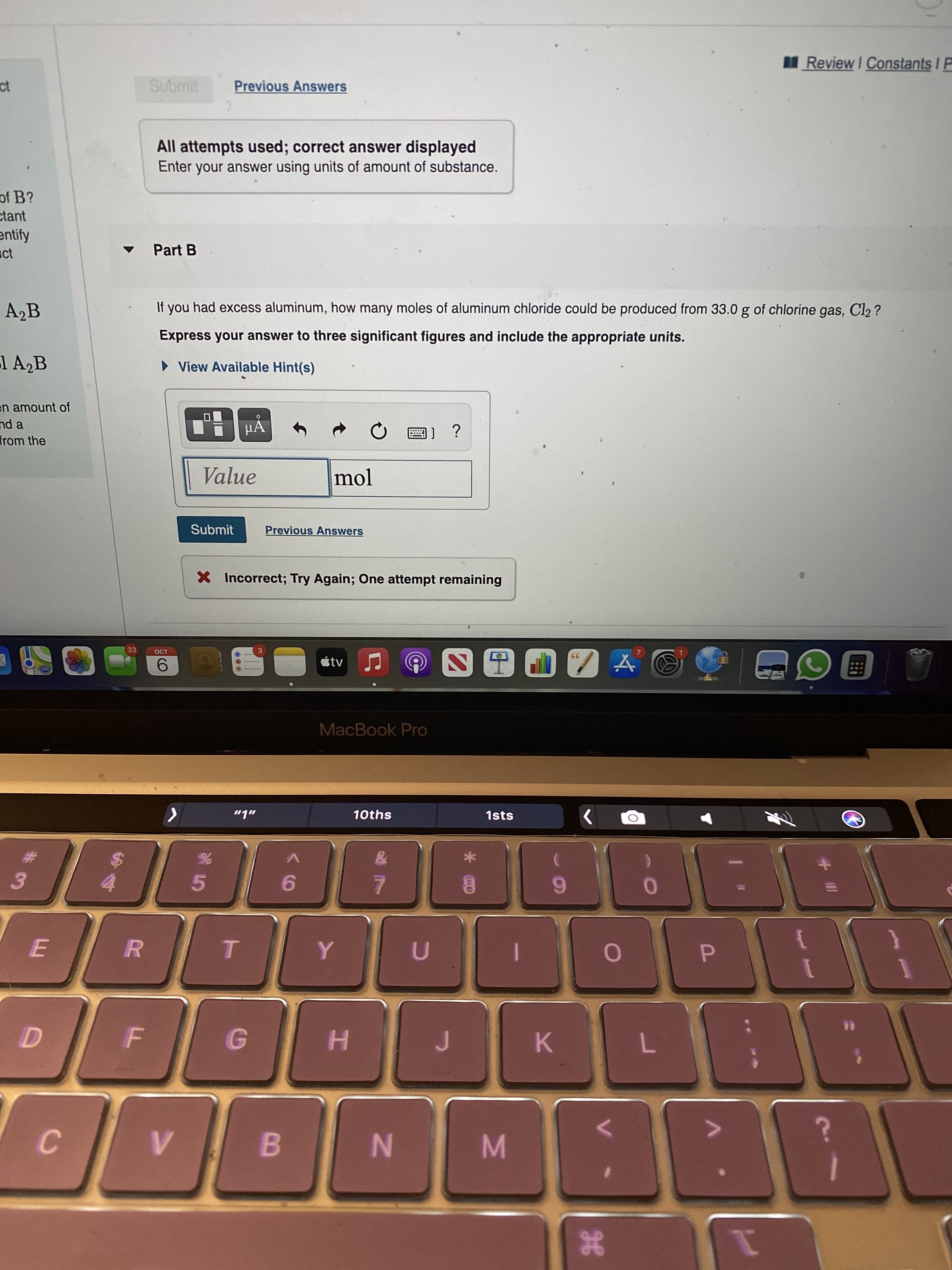
If you had excess aluminum, how many moles of aluminum chloride could be produced from 33.0 g of chlorine gas, Cl2 ? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) μΑ Value mol
If you had excess aluminum, how many moles of aluminum chloride could be produced from 33.0 g of chlorine gas, Cl2 ? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) μΑ Value mol
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 80AP: Using the average atomic masses given inside the front cover of the text, calculate the mass in...
Related questions
Question
100%
Transcribed Image Text:* 00
IN
%一
R
I Review I Constants I P
Submit
Previous Answers
All attempts used; correct answer displayed
Enter your answer using units of amount of substance.
of B?
ctant
entify
act
Part B
A,B
If you had excess aluminum, how many moles of aluminum chloride could be produced from 33.0 g of chlorine gas, Cl2 ?
Express your answer to three significant figures and include the appropriate units.
1A,B
• View Available Hint(s)
en amount of
nd a
irom the
Value
mol
Submit
Previous Answers
X Incorrect; Try Again; One attempt remaining
33
3.
étv
国
MacBook Pro
10ths
1sts
*
3.
5.
9.
E.
P.
K.
7.
C.
B.
58
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning