Enter the coefficients directly from the balanced chemical equation. Do not reduce to lowest terms. For example: If 3 moles H2 react with 3 moles O2, enter "3" for each, not "1". According to the following reaction: 2NO (g) + 2H2 (g) –→N2 (g)+ 2H2O (1) What would you multiply "moles of nitrogen monoxide" by to convert to the units "moles of water" ? (number) (unit) moles NO = moles H2O (number) (unit)
Enter the coefficients directly from the balanced chemical equation. Do not reduce to lowest terms. For example: If 3 moles H2 react with 3 moles O2, enter "3" for each, not "1". According to the following reaction: 2NO (g) + 2H2 (g) –→N2 (g)+ 2H2O (1) What would you multiply "moles of nitrogen monoxide" by to convert to the units "moles of water" ? (number) (unit) moles NO = moles H2O (number) (unit)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 81QRT: Write the balanced chemical equation for the complete combustion of adipic acid, an organic acid...
Related questions
Question
100%
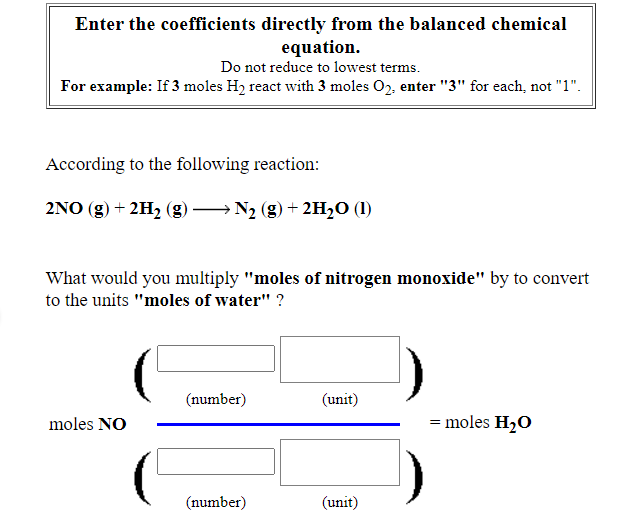
Transcribed Image Text:Enter the coefficients directly from the balanced chemical
equation.
Do not reduce to lowest terms.
For example: If 3 moles H2 react with 3 moles 02, enter "3" for each, not "1".
According to the following reaction:
2NO (g) + 2H2 (g)
→ N2 (g) + 2H20 (1)
What would you multiply "moles of nitrogen monoxide" by to convert
to the units "moles of water" ?
(number)
(unit)
moles NO
= moles H20
(number)
(unit)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div