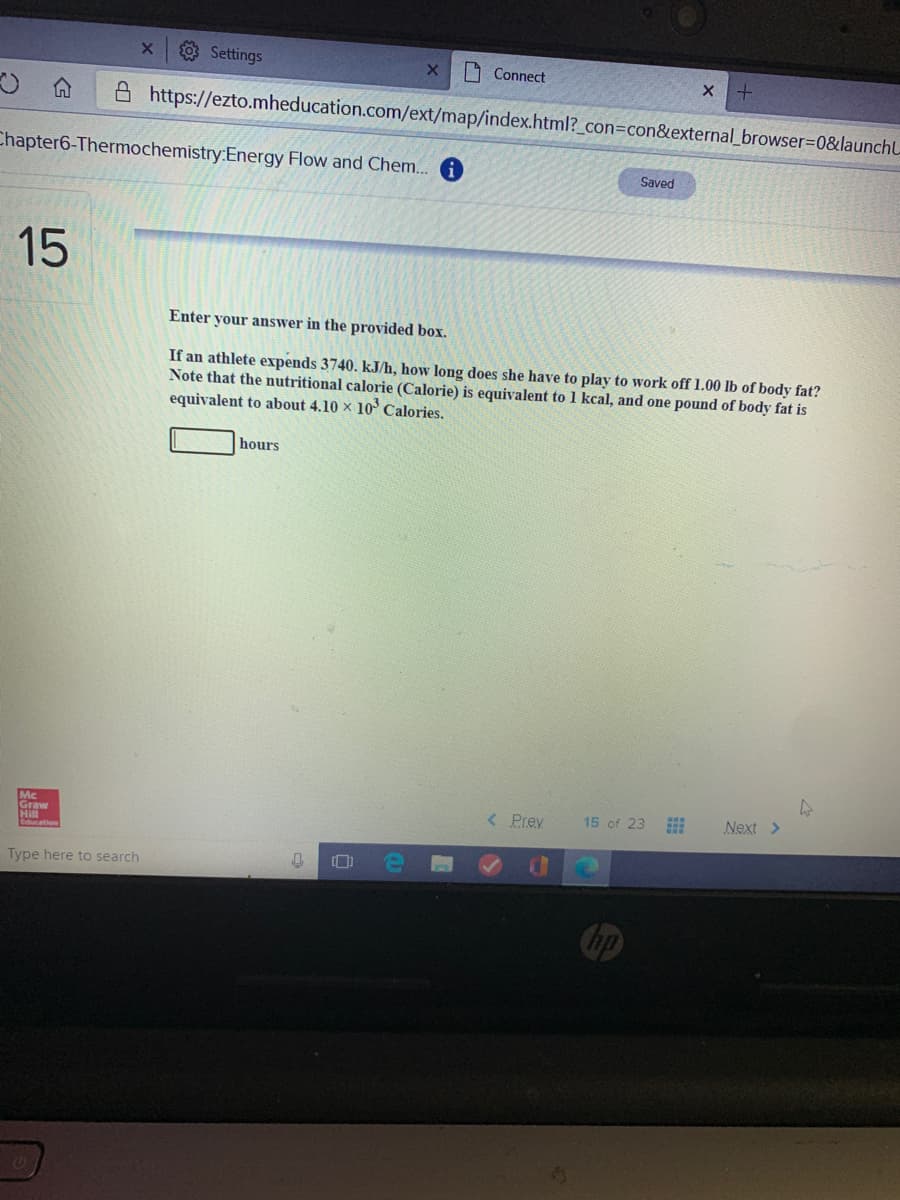
Enter your answer in the provided box. If an athlete expends 3740. kJ/h, how long does she have to play to work off 1.00 lb of body fat? Note that the nutritional calorie (Calorie) is equivalent to 1 kcal, and one pound of body fat is equivalent to about 4.10 × 10 Calories. hours
Enter your answer in the provided box. If an athlete expends 3740. kJ/h, how long does she have to play to work off 1.00 lb of body fat? Note that the nutritional calorie (Calorie) is equivalent to 1 kcal, and one pound of body fat is equivalent to about 4.10 × 10 Calories. hours
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.104PAE: 9.104 An engineer is using sodium metal as a cooling agent in a design because it has useful thermal...
Related questions
Question
Transcribed Image Text:O Settings
Connect
A https://ezto.mheducation.com/ext/map/index.html?_con=con&external_browser3D0&launchu
Chapter6-Thermochemistry:Energy Flow and Chem. 6
Saved
15
Enter your answer in the provided box.
If an athlete expends 3740. kJ/h, how long does she have to play to work off 1.00 lb of body fat?
Note that the nutritional calorie (Calorie) is equivalent to 1 kcal, and one pound of body fat is
equivalent to about 4.10 × 10° Calories.
hours
Mc
Graw
Hill
< Prev
15 of 23
Next >
Type here to search
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning