Q: Which metal requires large amounts of energy to reduce it from its ore, and can only be produced by…
A: Answer Aluminum All the other metals are transition metal and can be extracted using calcination…
Q: ne shape of the model molecule on
A:
Q: 5. Complete the following nuclear equation: Ca +, H → Sc+ 20 21 6. Complete the following nuclear…
A: In nuclear reaction decay of reactant is takes place. After decay different elements or isotope is…
Q: Use the Lewis dot structure to predict the electron domain geometry and molecular geometry for the…
A: To draw lewis dot structure , we need valance electrons of all atoms . Then we make required number…
Q: 10p in recent years, technology has developed considerably in analytical devices as in many areas…
A:
Q: What is the correct name of the molecule with the formnulas CH; CH, CH, CH, CH, CH2 CH3? Heptane…
A:
Q: Select the organic product(s) of the following reaction below. More than one answer may be selected.…
A:
Q: CH3 H3C-
A: Important points: Double bond give higher priority than alkyl group. When the alkyl substitent is…
Q: Part A For the hatl-reaction Cr"eCr, E- 0.424V excess Fe(s) is added to a solution in which (Cr"]…
A:
Q: What would be an appropriate term to describe molecules B and C? in A В Me `Me Me resonance…
A: Molecule A is conjugated. B and C are skip diene
Q: percentage of his original carbon-14
A:
Q: Lalaulate the eH of a 3 lox1D M la/OH),s Selution.CafoH)is. isa strory buse.
A:
Q: If the same mass of Na2SO4 (MW: 142 g/mol) and K2SO4 (MW: 174 g/mol) were weighed and used for the…
A: The balanced equation for the formation of the precipitate, BaSO4(s) is: Ba2+(aq) + SO42-(aq)…
Q: 3. Fill the boxes with the missing reactants, reagents, or the major product(s) expected from each…
A: Detail mechanistic pathway is given below to find out the correct product as well as missing reagent
Q: Instructions: į Rewrite the given information in the form of a reaction scheme*. - Draw the skeletal…
A: The reaction is an example of nucleophilic aromatic substitution reaction. The reaction proceeds via…
Q: Which statement best applies to the concept of corrosion Corrosion involves the reduction of a metal…
A: Answer: When a pure metal substance reacts with another substance and loses its natural forms, then…
Q: What could be the effect of the following solutions on the determination of the molecular weight of…
A: We have asked to answer what could be the effect of the following solutions on the determination of…
Q: Calculate the osmotic pressure induced if a cell with a total solute concentration of 0.500 moles…
A: Answer: Formula to calculate osmotic pressure is shown below: π=CRT Here: π=osmotic…
Q: what is acid-base equilibrium or electrochemistry? explain it using an illustration.
A: Acids and bases have a chemical equilibrium in solution. At chemical equilibrium, the products and…
Q: 3. Complete and balance the following single displacement equations. If no reaction takes place,…
A: The balanced single displacement reaction are given below
Q: Concentration of hydrochloric acid (M) 2.07 Concentration of sodium hydroxide (M) 2.09 Calorimeter…
A:
Q: 44.00 mL of 0.50 M H2S04 reacts with 0.35 M LIOH and reaches the endpoint using bromthymol blue as…
A: Recall the reaction, 2LiOH + H2SO4 ----> Li2SO4 + 2H2O Molarity of H2SO4=0.50 M , Volume=44.0 ml…
Q: Exercise 9.83 In the first chapter of this book, we described the scientific approach and put a…
A: # We can predict magnetic behaviour of any molecule by Lewis theory. If it has unpaired electrons,…
Q: What is the direction of anion movement in an electrochemical cell? O Anode to cathode through the…
A: we need to tell the direction of movement of anions in an electrochemical cell
Q: equation for the esterification of glycerol and three ethanoic acids
A: equation for the esterification of glycerol and three ethanoic acid is given below
Q: What is an emulsifier? Why do ice cream manufacturers put them in the mix? Explain using concepts…
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: A waste heat exchanger is used to absorb the energy from the complete combustion of hydroge sulfide…
A: The source below says: 519kg/mol (12kg H2S) * (1000g/kg)/(34.0814)*(519kj/mol)=228424KJ
Q: Solve for the pH of a 0.22 M AlCl3 (aq) solution. Ka of Al3+ is 1.2 × 10–5.
A: When we make aqueous solution of AlCl3 this undergo cationic hydrolysis and form hydrated aluminum…
Q: What is the equilibrium constant expression for the reaction shown below? CACO3(s) R Ca²+ (s)ɛ +…
A: What is the equilibrium constant expression for the given reaction---
Q: How much heat is needed to melt 10 g of ice?
A: formula for this is, heat = mass*latent heat of water as ice melts to water so water considered
Q: Write the Bronsted-Lowry reactions in aqueous solutions of the following substance: NaH2PO4(s).…
A: According to Bronsted Lowry definition of acid and base : Acid is a species which donate Proton…
Q: . Please list all expected peaks, bends, and stretches you would expect to see on IR spectra of…
A:
Q: Explain why diethyl ether's boiling point is lower than the boiling point of butan-1-ol. Support…
A: The organic compounds given are diethyl ether (C2H5OC2H5) butan-1-ol (CH3CH2CH2CH2OH)
Q: What is the oxidation number of Ca in Ca3N2? Choose the right answer in the given choices below:…
A:
Q: Which gas is not associated with the formation of acid rain? NO2 CONO SO3
A: ->Acid rain refers to rain fall and other forms of precipitation with a pH of less than 5 .…
Q: Trial 1 2 3 S Final Volume Reading NAOH (ml) 36.65 38.23 35.80 Initial Volume Reading NaOH (ml) Net…
A: Standardization of sodium hydroxide
Q: 4)Listen Which of the following best describes a chemical reaction that absorbs heat from the…
A:
Q: Define the following: 1. solvent 2. mixture 3. solution
A: (1) Solvent - Solution is formed by combination of atleast two Substances, one of them has higher…
Q: но O-H OCH,CH HO.
A:
Q: Consider the reaction: Cu2*(aq) + 4NH3(aq) Cu(NH3)4²*(aq) Kf = 1.7 x 1013 What is [Cu2+] remaining…
A: Each CuSO4 is dissociated in water to give 1 Cu2+(aq) ion. Hence moles of CuSO4 = moles of Cu2+(aq)…
Q: Instructions: į Rewrite the given information in the form of a reaction scheme*. - Place the main…
A: Since you have asked multiple questions we will answer the first one for you. To get the remaining…
Q: What pH a 4 3X 10 M HCI Sautidn ? HClisa strong cid. is the
A:
Q: What type of system is able to exchange both energy and matter with its surroundings?
A:
Q: List 3 common weak acids. Include both chemical formula and chemical name. 1. 2. 3.
A: Weak acid dissociate less in solution
Q: 30.0 mL ethanol to make a 5.88 of ethanol in butanol?
A:
Q: Explain the following observation and what it means about the mechanism of the process we…
A: In 1st 25%product, 13CO i s removed and in last product Me group is trans to 13CO and i middle one…
Q: Assume that you are given a solution that was prepared as follows: 2.50 g of NaNO3, 2.50 g of…
A: Given that - Mass of sodium nitrate, NaNO3 = 2.50 g Mass of calcium nitrate, Ca(NO3)2 = 2.50 g…
Q: Given the equilibrium: 2HCI H2 (g) + Cl2 (e) Keg = 3.7 predict the equilibrium conc. of all…
A: As per our guidelines we can only solve first three sub-parts. Please resubmit the other questions…
Q: Complete and balance the following half-reactions: Cl, CI HOBR HBr (acid solution) W,O, WO, (acid…
A: Here we have to balanced the following half reaction in acidic medium . Here to balance O atom we…
Q: If the energy of activation is lowered for a chemical reaction, which is true? OThe reaction…
A: Given that, What happened if energy of activation of a reaction is lowered?
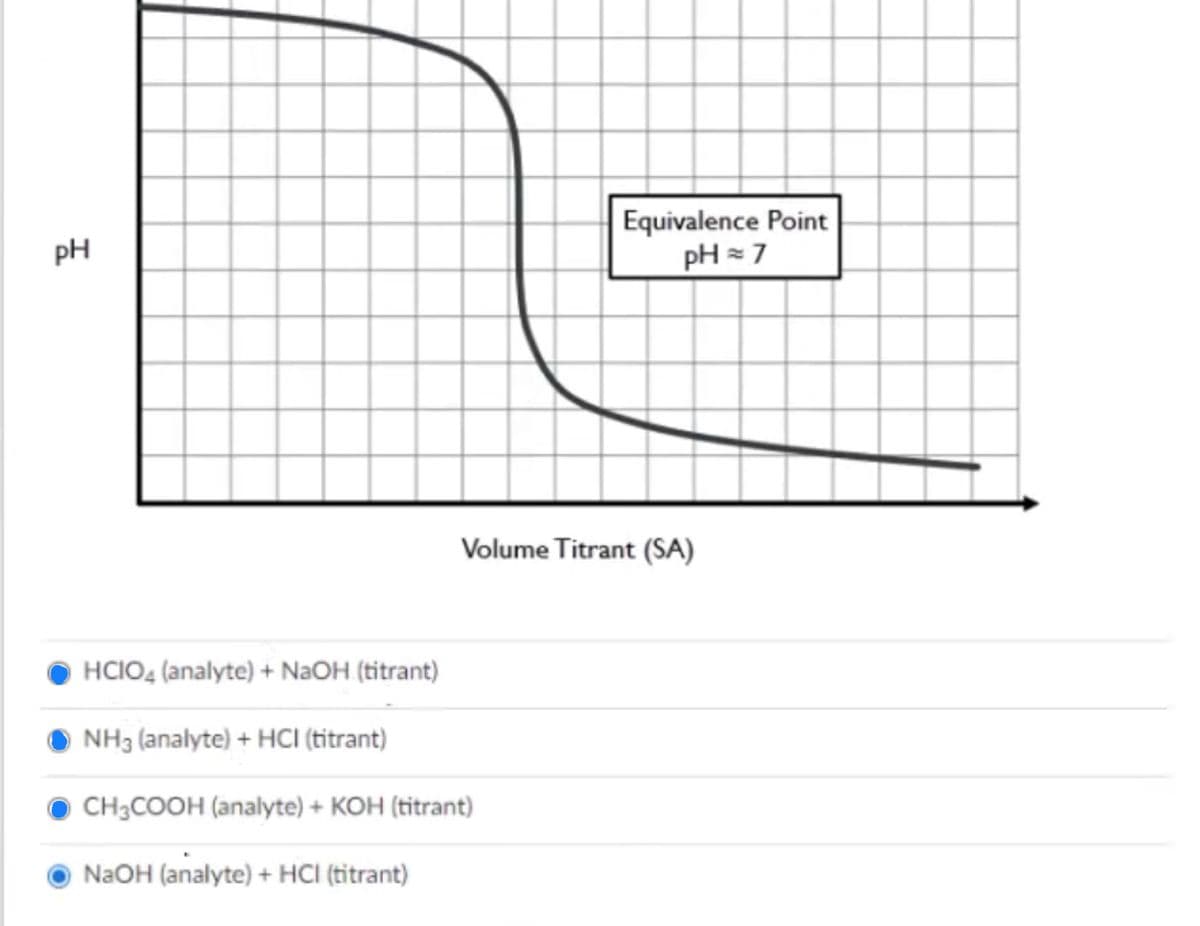
The following titration curve is expected from which acid-base pair?
See the picture below.
Step by step
Solved in 2 steps

- Express the concentration of acetic acid in both samples as % by mass of acetic per 100mL of solution given : %= mass/ volumex100% Given: NaOH vs CH3COOH Burette solution is NaOH and the pipette solution is 5.0 mL of Vinegar Titration Initial burette reading Final burette reading Volume of NaOH consumed Average volume of NaOH Approximate 0.0 mL 20.1 mL 20.1 mL 20.3 mL Titration 1 0.0 mL 20.9 mL 20.9 mL Titration 2 0.0 mL 20.0 mL 20.0 mL To find the average volume Average volume = 20.1 mL + 20.9 mL + 20.0 mL320.1 mL + 20.9 mL + 20.0 mL3 = 20.3 mL Concentration of Vinegar = Volume of NaOH * Concentration of NaOHVolume of vinegarVolume of NaOH * Concentration of NaOHVolume of vinegar = 20.3 mL * 0.0647 M5.0 mL20.3 mL * 0.0647 M5.0 mL = 0.2627 M Concentration of NaOH = Volume of H2SO4 * Concentration of H2SO4Volume of NaOHVolume of H2SO4 * Concentration of…NaOH + HCl ---> NaCl + H2O 123.60 mL of 3.90 M aqueous NaOH is required to titrate 346.00 mL of an aqueous solution of HCl to the equivalence point. The molarity of the analyte is 1.39 M. True or FalseIn the experiment conducted to determine the amount of acetic acid in vinegar, 8.2 mL of NaOH standard solution adjusted to 0.098 M was spent besides the phenolphthaleley indicator. Accordingly, which of the following is the amount of acetic acid in the vinegar sample in grams? (Ma (CH3COOH): 60.05 g / mol)
- Calculate the percent difference in the two values for the molarity of the NaOH solution by: %Difference =[M1-M2]\Mavg x100% Given: 1st value for the molarity of NaOH = 0.0647 M 2nd value for the molarity of NaOH= 0.2627 M NaOH vs H2SO4 Burette solution is NaOH and the pipette solution is 10.0 mL of 0.205 M H2SO4 Titration Initial burette reading Final burette reading Volume of NaOH consumed Average volume of NaOH Approximate 0.0 mL 31.8 mL 31.8 mL 31.7 mL Titration 1 0.0 mL 31.5 mL 31.5 mL Titration 2 0.0 mL 31.7 mL 31.7 mL To find the average volume Average volume = 31.8 mL + 31.5 mL + 31.7 mL331.8 mL + 31.5 mL + 31.7 mL3 = 31.7 mL NaOH vs CH3COOH Burette solution is NaOH and the pipette solution is 5.0 mL of Vinegar Titration Initial burette reading Final burette reading Volume of NaOH consumed Average volume of NaOH Approximate 0.0 mL 20.1 mL 20.1 mL 20.3 mL Titration 1 0.0 mL 20.9 mL 20.9 mL Titration 2 0.0 mL 20.0 mL…What is the pH of the solution after mixing 0.171 g of Mg(OH)2 (MW=58.321 g/mol) with 68.9 mL of 0.0569 M HCl? The resulting solution was diluted to 100 mL. Round your calculated value for pH to two figures to the right of the decimal point.The weak monoprotic acid, acetic acid, is titrated with the strong base, potassium hydroxide as follows: HC2H3O2(aq) + K+ OH- (aq) → K+ C2H3O2-(aq) + H2O(l) Ka for acetic acid is 1.81 x 10-5 (at 25 oC). A 25.00 mL sample of a solution of acetic acid with concentration 0.0833 M is titrated with 0.1000 M KOH. A. What is the pH at the beginning of the titration, Vbase = 0.00 mL? B. What is the pH at the equivalence point? C. What is the pH of the titration when 5.00 mL of base have been added? D. What is the pH when the volume of base added equals half the volume of the equivalence point? E. What is the pH of the titration when 20.00 mL of base have been added? F. What is the pH of the titration when 30.00 mL of base have been added?
- 5 SO2 + 2 MnO4- + H2O ---> 5 SO42- + 2 Mn2+ + 4 H+ The MnO4- solution is poured in a burette and then titrated till the equivalent point is aquired. How many L are needed of MnO4? (the burette starts at 5,7 and is 37,3 after titration)A hydrochloric acid solution is standardized by titrating 0.5008 g of primary standard tris(hydroxymethyl) aminomethane, C4H11NO3, with 99.9780% purity. If 39.68 mL isrequired for the titration, what is the molarity of the acid?In the tiration of what base with KHP (endpoint) can we use "moles of base= 2 x moles of KHP" equivalence ?
- The weak monoprotic acid, acetic acid, is titrated with the strong base, potassium hydroxide as follows: HC2H3O2(aq) + K+ OH- (aq) → K+ C2H3O2-(aq) + H2O(l) Ka for acetic acid is 1.81 x 10-5 (at 25 oC). A 25.00 mL sample of a solution of acetic acid with concentration 0.0833 M is titrated with 0.1000 M KOH. a) Sketch the pH vs volume of added base titration curve for this reaction. pH vertically, volume of base horizontally. Label your axes with correct pH and volumes. (Attach more space, if needed) b) what is the pH at the beginning of the titration, Vbase = 0.00 mL ? c) what is the volume of the base needed to reach the equivalence point ? (label the equiv. point) d) what is the pH at the equivalence point?e) what is the pH of the titration when 5.00 mL of base have been added ? f) what is the pH when the volume of base added equals half the volume of the equivalence point? g) what is the pH of the titration when 20.00 mL of base have been added? h) what is the pH of the titration…Molarity of (NH3) solution (M) from bottle- 5.0 Initial reading of buret (NH3) (mL)- .27 Final reading of buret (NH3) (mL)- 8.25 Volume of Cd(NO3)2 solution (mL)- 10.00 Volume of Na2C2O4 solution (mL)- 10,00 please find Total volume of solution after titration (mL) Total moles of C2O42- (mol) Molarity of C2O42- (M) Total moles of Cd2+ (mol) Moles of [Cd(NH3)4]2+ (mol) Molarity of [Cd(NH3)4]2+ (M) Moles of NH3 added by titration (mol) Moles of NH3 that did not react with Cd2+ (mol) Molarity of NH3 that did not react with Cd2+ (M) Kf for [Cd(NH3)4]2+16.The titration of an aliquot of 6 mL of acetic acid of commercial use, with NaOH 0.1 , was made by titration, so that to reach the equivalence point 40 ml of NaOH were consumed.Determine the percentage concentration (m/m) of the acetic acid, if its density is 1.05 g/ml. HC2H3O2 acetic acid

