Trial 1 2 3 S Final Volume Reading NAOH (ml) 36.65 38.23 35.80 Initial Volume Reading NaOH (ml) Net Volume NAOH used (ml) 0.00 0.50 0.00 Calculation of standardized Molarity of NaOH via dimensional analysis Trial 1:
Trial 1 2 3 S Final Volume Reading NAOH (ml) 36.65 38.23 35.80 Initial Volume Reading NaOH (ml) Net Volume NAOH used (ml) 0.00 0.50 0.00 Calculation of standardized Molarity of NaOH via dimensional analysis Trial 1:
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
Fill in the following table using the information given.

Transcribed Image Text:Trial
1
2
3
Final Volume Reading NaOH (ml)
36.65
38.23
35.80
Initial Volume Reading NAOH (ml)
0.00
0.50
0.00
Net Volume NaOH used (ml)
Calculation of standardized Molarity of NAOH via dimensional analysis Trial
1:
Trial 2:
Trial 3:
Calculation of average Molarity of NAOH
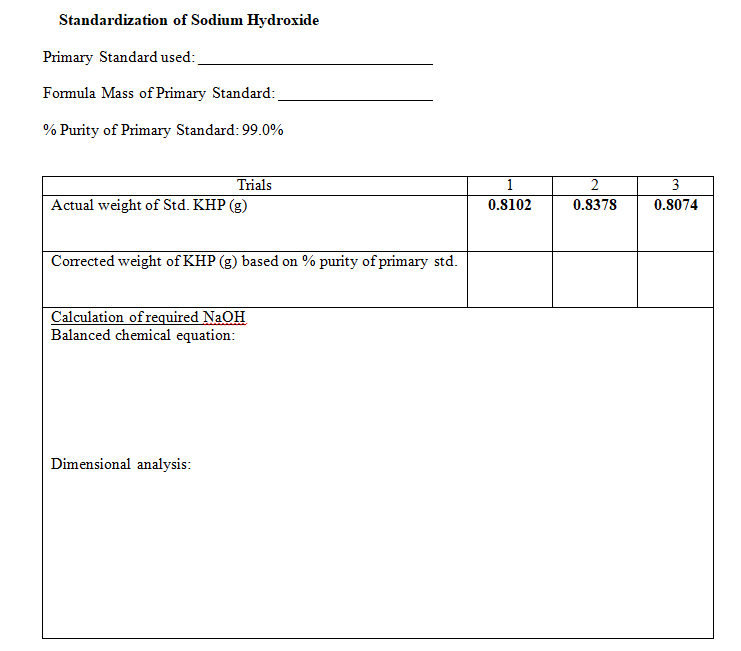
Transcribed Image Text:Standardization of Sodium Hydroxide
Primary Standard used:
Formula Mass of Primary Standard:
% Purity of Primary Standard: 99.0%
1
0.8102
Trials
2
Actual weight of Std. KHP (g)
0.8378
0.8074
Corrected weight of KHP (g) based on % purity of primary std.
Calculation of required NAOH
Balanced chemical equation:
Dimensional analysis:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning