es Lx Give Up? Y Hint For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species. For the reaction N,(g) + 3 H, (g) =2 NH,(g) the standard change in Gibbs free energy is AG° = -32.8 kJ/mol. What is AG for this reaction at 298 K when the partial %3D pressures are PN, = 0.100 atm, PH, = 0.300 atm, and PNH, = 0.650 atm? %3D %3D kJ/mol AG = TOOLS x10 about us careers privacy policy terms of, use contact us help
es Lx Give Up? Y Hint For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species. For the reaction N,(g) + 3 H, (g) =2 NH,(g) the standard change in Gibbs free energy is AG° = -32.8 kJ/mol. What is AG for this reaction at 298 K when the partial %3D pressures are PN, = 0.100 atm, PH, = 0.300 atm, and PNH, = 0.650 atm? %3D %3D kJ/mol AG = TOOLS x10 about us careers privacy policy terms of, use contact us help
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 83QAP
Related questions
Question
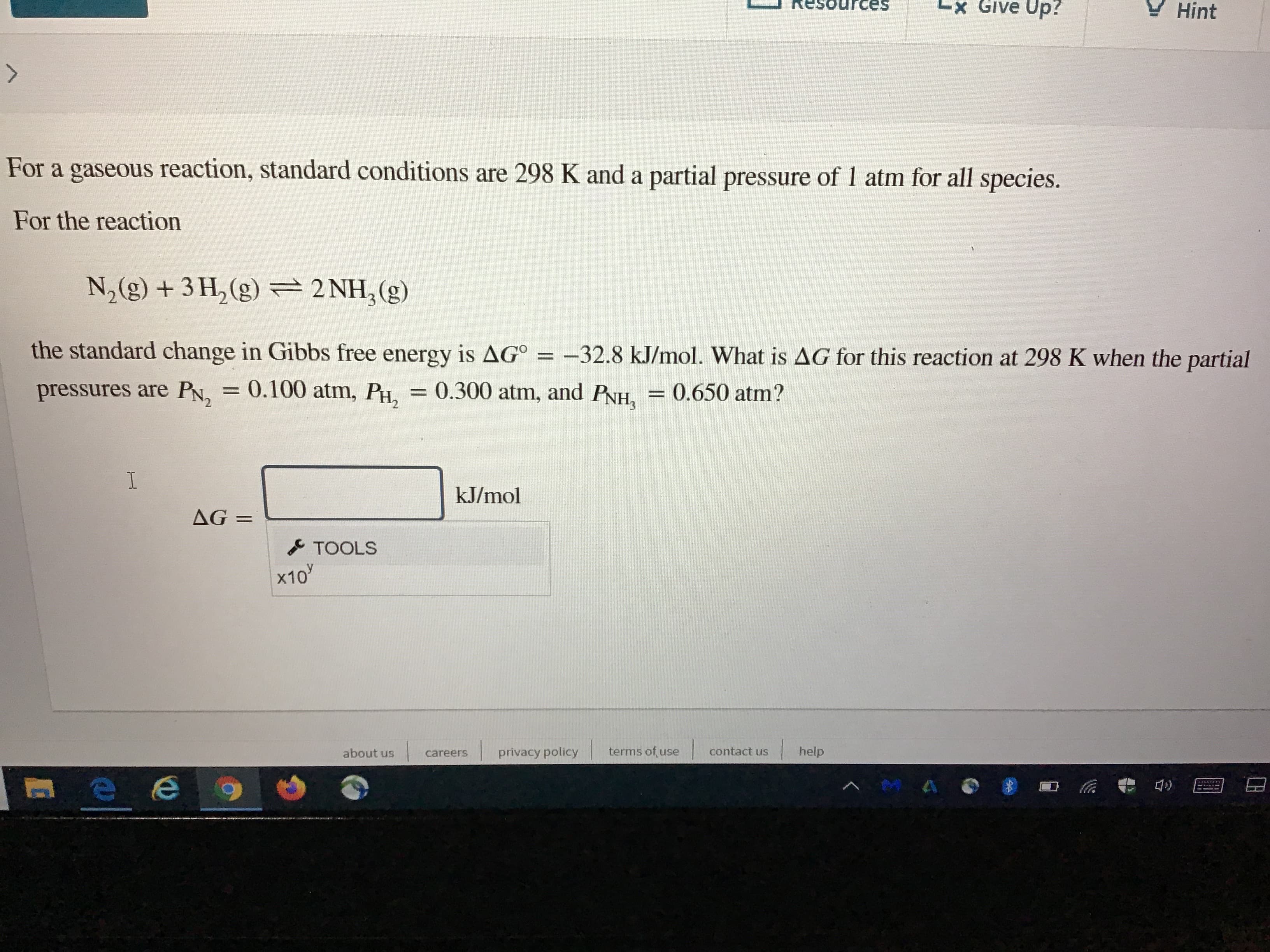
Transcribed Image Text:es
Lx Give Up?
Y Hint
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species.
For the reaction
N,(g) + 3 H, (g) =2 NH,(g)
the standard change in Gibbs free energy is AG° =
-32.8 kJ/mol. What is AG for this reaction at 298 K when the partial
%3D
pressures are PN, = 0.100 atm, PH,
= 0.300 atm, and PNH,
= 0.650 atm?
%3D
%3D
kJ/mol
AG =
TOOLS
x10
about us
careers
privacy policy
terms of, use
contact us
help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning