Q: A student titrated 50.0mL of a 0.10 M solution of a certain weak acid with NaOH(aq), what is the…
A: Step 1: From the given graph, it is clear that pH range 6 to 10 is found at 25.0 mL of NaOH added.…
Q: A weak acid has Ka of 4.47 x 10-4. What is pKa for this weak acid?
A:
Q: Which one of the following indicators is best suited for titrating 0.05 M pyridine (pKb = 8.82) with…
A:
Q: What is the pH when 1 mole of HCl is added to 1 mole of Na3AsO4 (arsenic acid) in a 1 liter volume?…
A: Na3AsO4 is sodium salt of arsenic acid. So when HCl is added to it , they will react to give…
Q: 10.00 mL sample of 1.20 M HNO2 (analyte) (pKa = 3.34 and Ka = 4.57x10-4) is titrated with 1.20 M…
A: Given , pka = 3.34 Molarity of HNO2 = 1.20 M Volume of HNO2 = 10 mL Mmol of HNO2 = M × V = 1.20…
Q: In titrating 10 ml of 0.1 N HA (pKa-4),the pH after the addition of 12 ml 0.1N NaOH is O 3.69 О 11.2…
A: Given Normality of HA = 0.1 N Volume of HA = 10 mL Mole of HA = Normality of HA * Volume of HA Mole…
Q: 14. What is the pH of a mixture of 0.20M acetic acid and 0.30M sodium acetate, if the pKa of the…
A: Dear student , since you have posted multiple questions we will allow to solve only first question…
Q: 1.Calculate the change in ph when 5ml of 1M HCl solution is added to 1L of 100mM Tris solution at ph…
A:
Q: Which one of the following indicators is best suited for titrating 0.40 M ammonia (pKb = 4.74) with…
A: The chemical reaction occurs in the given titration (strong acid+weak base) is: NH3 + HCl = NH4Cl…
Q: Which of the following titration systems will have the largest ApH at the equivalence point region?…
A: In acid-base titration is an example of neutralization reaction, so the pH at equivalence point is…
Q: The acid solution is known to contain 3 moles of HA with a constant of Ka and 1 mol of Na. Of 1 mole…
A:
Q: You have been asked to prepare 1 L of a 100 mM TRIPS (pKa 6.9) buffer. How much should you take of…
A: From the Henderson Hasselbalch equation pH = pKa + log [salt]/[acid] The base used to make TRIS…
Q: An analytical chemist is titrating 190.8 mL of a 0.4600M solution of nitrous acid (HNO,) with a…
A: The titration reaction between Nitrous acid and KOH can be represented as- HNO2 + KOH ----> KNO2…
Q: An analytical chemist is titrating 134.4mL of a 0.6500M solution of nitrous acid HNO2 with a 0.7300M…
A: The molarity of an unknown solution can be determined by titrating it with a known solution by using…
Q: During the titration of 25 mL of 0.1 M maleic acid with 0.1M NAOH. We have studied the plot of alpha…
A: The graph of alpha values and Volume is as follows:
Q: Describe how to prepare 0.500 L of 0.100 M imidazole buffer, pH=7.50, pKa= 6.99 starting with…
A: Henderson-Hasselbach equation shows the relationship between pH of a solution, the acid dissociation…
Q: 8. Phosphoric acid (H3PO4) can give up three protons, each with different pKa values consisting of…
A: Given As H3PO4 a tri-protic acid. it will show 3 equivalence point H3PO4…
Q: How do I set up the equation to figure out the concentrations of HA and OH-?
A: A buffer is a solution that can resist any change in pH upon addition of small amounts of acid or…
Q: Which of the following is the best buffer at neutral pH (pH 7.0)? O Water, pKa = 14 Phosphoric acid,…
A: The question is based on the concept of pH of the solution. it is defined as a negative logarithm…
Q: An analytical chemist is titrating 160.2 mL of a 0.3300M solution of nitrous acid (HNO,) with a…
A: Given: The reaction is as follows: HNO2(g) + KOH(l) ⇌ KNO2(aq) + H2O. The volume of HNO2, (V1) =…
Q: An analytical chemist is titrating 97.8 mL of a 1.100M solution of nitrous acid (HNO,) with a…
A:
Q: Use the Henderson-Hasselbach equation to determine the ratio of acid to conjugate base if a buffer…
A:
Q: A hypothetical weak acid, HA, was combined with NaOH in the following proportions: 0,20 mol HA, 0,08…
A:
Q: Calculate the Ka and Kb values for 1.0 M NaHSO4 and Na2CO3. Expression for Kb and Value of Kp and…
A: pH = - log [H+] Kw = Ka X Kb pH + pOH = 14
Q: Consider the list of acids and their pKa values to the right. What two compounds would be best to…
A: Buffers are effective when the pKa values are closest to pH.
Q: What is the pH of a solution made by titrating 265. mL of 0.014 M propanoic acid (Ka = 1.34x10-5)…
A: Propanoic acid is the weak acid so pH of given titrating mixture is calculated as follows, Given.…
Q: Phosphorous acid, H,PO,(aq), is a diprotic oxyacid that is an important compound in industry and…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: An analytical chemist is titrating 159.5 mL of a 1.200 M solution of nitrous acid (HNO,) with a…
A: The is a neutralization reaction which takes place as,…
Q: In a lab, you titrate 27.0 mL of 0.100 M acetic acid (CH3COOH, a weak acid, pKa 5.756, Ka 2.75 x…
A:
Q: A sodium hydrogen carbonate/sodium carbonate buffer system is to be prepared with a pH of 9.89. The…
A: The question is based on the concept of buffer solution. A buffer is a solution which resist any…
Q: 1) Using the measured pH, calculate the concentration of H3O+ and OH- at the equivalence point.
A: As per regulations we are only supposed to answer only one question.
Q: Dissolve Tris (FW=121.14 g, pKa=8.07) 12.43 g and Tris.HCl (FW=157.60 g) in water, add 12.0mL of…
A: The question is based on the concept of buffer solution. A buffer is a solution which resist any…
Q: Which solution is closest to the pKa? Calculate the pH of the solutions and the change in pH after…
A: Since you have posted a question with multiple sub-parts, we will solve first three subparts for…
Q: Which of the following weak acids when paired with its conjugate base would be the best choice for a…
A: Given group of answer choices : a). HClO: pKa = 7.54 b). C5H4NH+: pKa = 5.25 c). HNO2: pKa =…
Q: 125 mL of a 0.109 M phenylalanine (pKal = 1.83, pKa2 = 9.13) solution is titrated with 0.223 M NaOH.…
A: Since phenylalanine is a weak acid with pKa1 = 1.83 and pKa2 = 9.13 And since pKa1 >> pKa2…
Q: Calculate the pH of a 1.00L solution containing 0.52M sodium acetate and 0.48M acetic acid after the…
A: Given that, concentration of CH3COONa in 1 L solution = 0.52 M = 0.52 mole L-1 and concentration of…
Q: I have 3 moles of a monoprotonated base (there are four pKa values(pKa1=2.3, pKa2=4.6,pKa3=10.1,…
A: The chemical reaction for the monoprotonated base can be given as follows: HCl+B-→HB+Cl-;…
Q: A monoprotic weak acid (HA) has a pKa value of 4.896. Calculate the fraction of HA in each of its…
A: The solution is given below -
Q: Harriet Rowki prepared 100.0 mL of 2.50 M H3BO3-NaH2BO3 buffer solution (pH = 8.50). The pKa value…
A: A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate…
Q: Which of the following is the best buffer at neutral pH (pH 7.0)? a) Acetic acid, pKa = 4.76 b)…
A: We know that, buffer zone is from ( pH - 1 ) to ( pH + 1 ) and at pH = pka buffer capacity maximum.…
Q: Identify the the weak acid-base equilibrium that corresponds to this Ka. When the analyte (acetic…
A: The question is based on the concept of titrations. we are titrating a weak acetic acid with a…
Q: Calculate the pH of the solution after the addition of each of the given amounts of 0.0603 M HNO3…
A: Given, Molarity of HNO3 = 0.0603 M Volume of aziridine (say B) in mL = 60.0 mL Molarity of…
Q: During an experiment, a student mixed 14.5 mL of a 0.500 M sodium fluoride solution with 15.6 mL of…
A: Given that : Volume of Sodium fluoride (NaF) = 14.5 mL = 0.0145L Concentration of Sodium fluoride =…
Q: The plot shows an oxygen-binding curve for human hemoglobin. Y (fractional saturation) 1.0 0.5 0 I 2…
A: Oxygen binding curve:--This curve shows the binding of oxygen to haemoglobin and tells about…
Q: Calculate the volume, in milliliters, of a 0.770 M KOH solution that should be added to 5.000 g of…
A: Given, Molarity of KOH = 0.770 M Mass of HEPES = 5 g Molecular weight = 238.306 g/mol pKa = 7.56…
Q: An analytical chemist is titrating 64.6 mL of a 0.8000 M solution of nitrous acid (HNO₂) with a…
A: Given data : Concentration of HNO2 = 0.8000 M Volume of HNO2 = 64.6 mL CConcentration of KOH =…
Q: Using the Henderson-Hasselbalch equation determine the ratio of conjugate base (HPO42–) to acid…
A: pH = 6.90 pKa = 7.20 Acid is H2PO4- and symbolized as HA Conjugate base is HPO42- and symbolized as…
Q: Which one of the following indicators is best suited for titrating 0.05 M methylamine (pK, = 3.19)…
A: Answer is explained below. In the given problem, there is a reaction between methylamine (weak base)…
Q: It takes 12.45 mL of 0.50 M NaOH to back titrate a mixture containing 1.25 g of baking soda, NAHCO…
A: Antacids are the substances which have tendency to neutralize acids.Technically, these are alkaline…
Q: A 10.00 mL sample of 1.20 M HNO2 (analyte) (pKa = 3.34 and Ka = 4.57x10-4) is titrated with 1.20 M…
A: Given, A 10.00 mL sample of 1.20 M HNO2 (analyte) (pKa = 3.34 and Ka = 4.57x10-4) is titrated with…
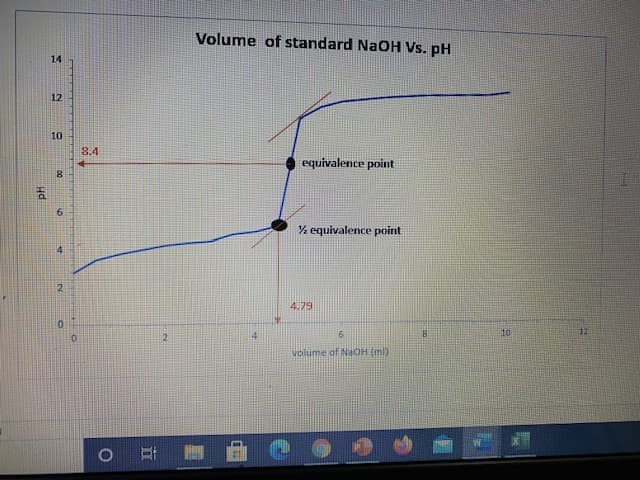
describe the curve and why pH = pka at half equivalence point
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- What happen to the separation raid (RS) I change the mobile phase from methanol/water 70/30 till methanol/water 80/20. How does polarity affect?draw and sketch a group for action poyential polarization phases with membrane potential vm in milivoltsExample: Calculation the weight of barium iodate Ba(IO3)2 if it dissolved in 500mL of distilled water (D.W) at 25 °C if M.wt of ppt = 487 g/mole and %3D Ksp= 1.57x10.
- What is the gravimetric factor if a weight of cl is to be determined from a weight of PbCl2?1. What is the Brownian movement? 2. What is the effect of Brownian movement to diffusion?Comelli and Francesconi examined mixtures of propionic acid with various other organic liquids at 313.15 K (F. Comelli and R. Francesconi, J. Chem. Eng. Data 41, 101 (1996)). They report the excess volume of mixing propionic acid with tetrahydropyran (THP, oxane) as VE = x1x2{a0 + a1(x1 − x2)}, where x1 is the mole fraction of propionic acid, x2 that of THP, a0 = −2.4697 cm3 mol−1, and a1 = 0.0608 cm3 mol−1. The density of propionic acid at this temperature is 0.971 74 g cm−3; that of THP is 0.863 98 g cm−3. (a) Derive an expression for the partial molar volume of each component at this temperature. (b) Compute the partial molar volume for each component in an equimolar mixture.
- In calculating ΔHmix for regular solutions, please derive 1) the mole fraction for the minimum of ΔHmix 2) the equation for the minimum of ΔHmixTo verify Beer’s Law for solution of KMnO4 or K2Cr2O7 using colorimeter.1 At a temperature of 302.8 K, mixtures of phenol andwater exist as two separate phases with mole fractionsof phenol of 0.30 and 0.93. Use the lever rule to determine the relative amounts of the two phases for amixture w ith an overall mole fraction of phenol of 0.45.
- The vapor pressures of 1,2-dibromoethane (M = 187.9 g mol-1and 1,2-dibromopropane (M =201.9 g mol-1), both measured at 343 K, are 12.90 and 9.17 kPa, respectively. Assuming thatthese two liquids form an ideal mixture, calculate the pressure of the vapor phase which is inequilibrium at 343 K with a liquid containing 50 % by mass of each of these componentsThe density of the solution prepared by dissolving 53.85 g of calcium chloride in 100 g of water at 18 C was measured as 1.3420 g/cm3. Calculate the mass fraction, mole fraction, molasses, molarity and normality of the dissolution.The freezing-point of depression of a 0.010 b acetic acid solution is 0.00193 K. Calculate thedegree of dissociation for acetic acid at this concentration. Cryoscopic constant for the aceticacid is 1.86 K mol kg mol-1.

