Estimate the percent ionic character of the bond in each of the following species. All the species are unstable or reac- tive under ordinary laboratory conditions, but they can be observed in interstellar space. Bond Length (À) Dipole Moment (D) OH 0.980 1.66 CH 1.131 1.46 CN 1.175 1.45 1.246
Estimate the percent ionic character of the bond in each of the following species. All the species are unstable or reac- tive under ordinary laboratory conditions, but they can be observed in interstellar space. Bond Length (À) Dipole Moment (D) OH 0.980 1.66 CH 1.131 1.46 CN 1.175 1.45 1.246
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.89QE
Related questions
Question
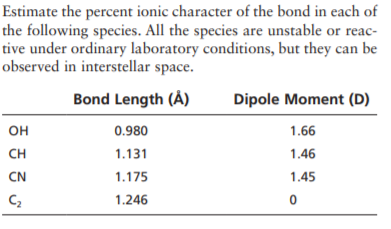
Transcribed Image Text:Estimate the percent ionic character of the bond in each of
the following species. All the species are unstable or reac-
tive under ordinary laboratory conditions, but they can be
observed in interstellar space.
Bond Length (À)
Dipole Moment (D)
OH
0.980
1.66
CH
1.131
1.46
CN
1.175
1.45
1.246
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning