Ethanol is the liquid you will be using in this weeks lab. There is a structural isomer of ethanol (a differnt molecule with the same formula) called dimethyl ether The molecules are below. Which choice correctly predicts the comapison of the vapor pressure of dimethyl ether to ethanol at room temperature based on intermolecular forces. (Picture attached) Select one: a. dimethyl ether will have a lower vapor pressure because it doesn't hydrogen bond. b. dimethyl ether will have a higher vapor pressure because it doesn't hydrogen bond. c. dimethyl ether will have a lower vapor pressure because it is larger and has more intermolecular forces. d. dimethyl ether will have the same vapor pressure because it has the same intermolecular forces as ethanol
Ethanol is the liquid you will be using in this weeks lab. There is a structural isomer of ethanol (a differnt molecule with the same formula) called dimethyl ether The molecules are below. Which choice correctly predicts the comapison of the vapor pressure of dimethyl ether to ethanol at room temperature based on intermolecular forces. (Picture attached) Select one: a. dimethyl ether will have a lower vapor pressure because it doesn't hydrogen bond. b. dimethyl ether will have a higher vapor pressure because it doesn't hydrogen bond. c. dimethyl ether will have a lower vapor pressure because it is larger and has more intermolecular forces. d. dimethyl ether will have the same vapor pressure because it has the same intermolecular forces as ethanol
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.2: Cis–trans Isomerism In Cycloalkanes
Problem 5P: Draw the structures of the following molecules: (a) trans-l-Bromo-3-methylcyclohexane (b) cis-1,...
Related questions
Question
100%
Ethanol is the liquid you will be using in this weeks lab. There is a structural isomer of ethanol (a differnt molecule with the same formula) called dimethyl ether The molecules are below. Which choice correctly predicts the comapison of the vapor pressure of dimethyl ether to ethanol at room temperature based on intermolecular forces. (Picture attached)
Select one:
a. dimethyl ether will have a lower vapor pressure because it doesn't hydrogen bond.
b. dimethyl ether will have a higher vapor pressure because it doesn't hydrogen bond.
c. dimethyl ether will have a lower vapor pressure because it is larger and has more intermolecular forces.
d. dimethyl ether will have the same vapor pressure because it has the same intermolecular forces as ethanol
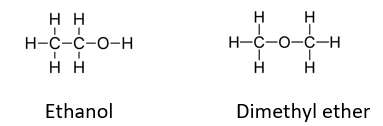
Transcribed Image Text:нн
Н-с-с-о-н
H-C-0-C-H
нн
H
Ethanol
Dimethyl ether
I-0-I
I-U-I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning