Even in basic solution, MnO4 can oxidize water. One product is manganese(IW oxide. Write a balanced chemical equation for the reaction 302(g) + 40H (aq) a. 2MNO4 (aq) + 2H20(1) ---> 2MN²*(aq) + b. 4MNO4 (aq) + 2H20() ---> 4MNO2(s) + 302(g) + 40H"(aq) С. MnO4 (aq) + H20(1) --> MnO2(s) + H2(g) + OH"(aq) d. 4MNO4 (aq) + H20() ---> 4MNO(s) + O2(g) + 20H"(aq) e. MnO4 (aq) + 6H20(1) ---> MnO2(s) + 2H2(g) + 80H (aq)
Even in basic solution, MnO4 can oxidize water. One product is manganese(IW oxide. Write a balanced chemical equation for the reaction 302(g) + 40H (aq) a. 2MNO4 (aq) + 2H20(1) ---> 2MN²*(aq) + b. 4MNO4 (aq) + 2H20() ---> 4MNO2(s) + 302(g) + 40H"(aq) С. MnO4 (aq) + H20(1) --> MnO2(s) + H2(g) + OH"(aq) d. 4MNO4 (aq) + H20() ---> 4MNO(s) + O2(g) + 20H"(aq) e. MnO4 (aq) + 6H20(1) ---> MnO2(s) + 2H2(g) + 80H (aq)
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 29Q: When jump-starting a car with a dead battery, the ground jumper should be attached to a remote part...
Related questions
Question
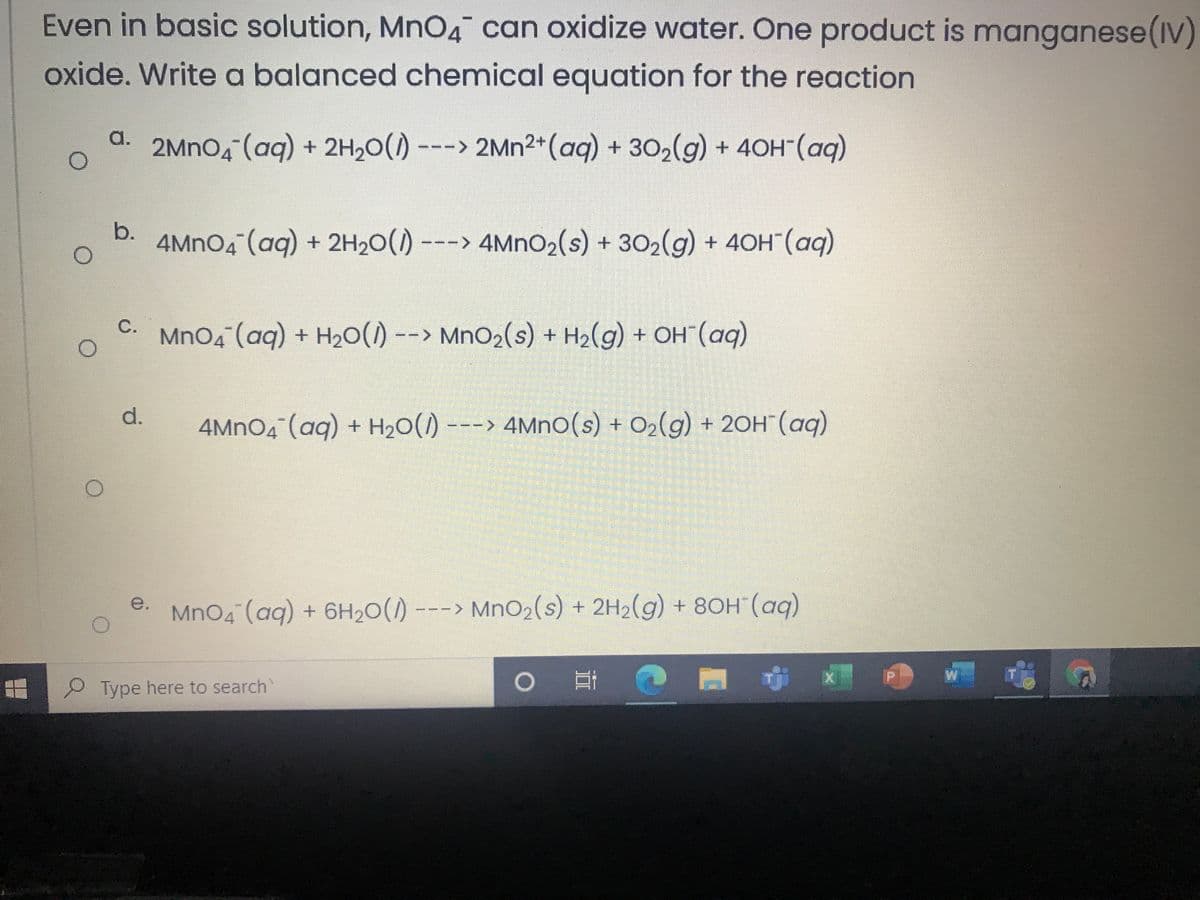
Transcribed Image Text:Even in basic solution, Mn04 can oxidize water. One product is manganese(IV)
oxide. Write a balanced chemical equation for the reaction
a.
2MNO4 (aq) + 2H20() ---> 2Mn2+(aq) + 302(g) + 40H (aq)
b.
4MNO4 (aq) + 2H20() ---> 4MNO2(s) + 302(g) + 40H"(aq)
MnO4 (aq) + H20() --> MnO2(s) + H2(g) + OH¯(aq)
d.
4MNO4 (aq) + H20() ---> 4Mno(s) + O2(g) + 20H (aq)
- > MnO2(s) + 2H2(g) + 80H (aq)
e.
MnO4 (aq) + 6H20(1)
市 @
Type here to search
C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning