Ex 1: A 14.81 g sample of a zinc sulfate hydrate, ZnSO4•xH;O(s), is heated to dryness leaving 8.27 g of the anhydrous salt, ZNSO4. (a) What is the chemical formula of the hydrate?
Ex 1: A 14.81 g sample of a zinc sulfate hydrate, ZnSO4•xH;O(s), is heated to dryness leaving 8.27 g of the anhydrous salt, ZNSO4. (a) What is the chemical formula of the hydrate?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter6: Solutions And Colloids
Section: Chapter Questions
Problem 6.50P
Related questions
Question
What is the chemical formula of the hydrate?
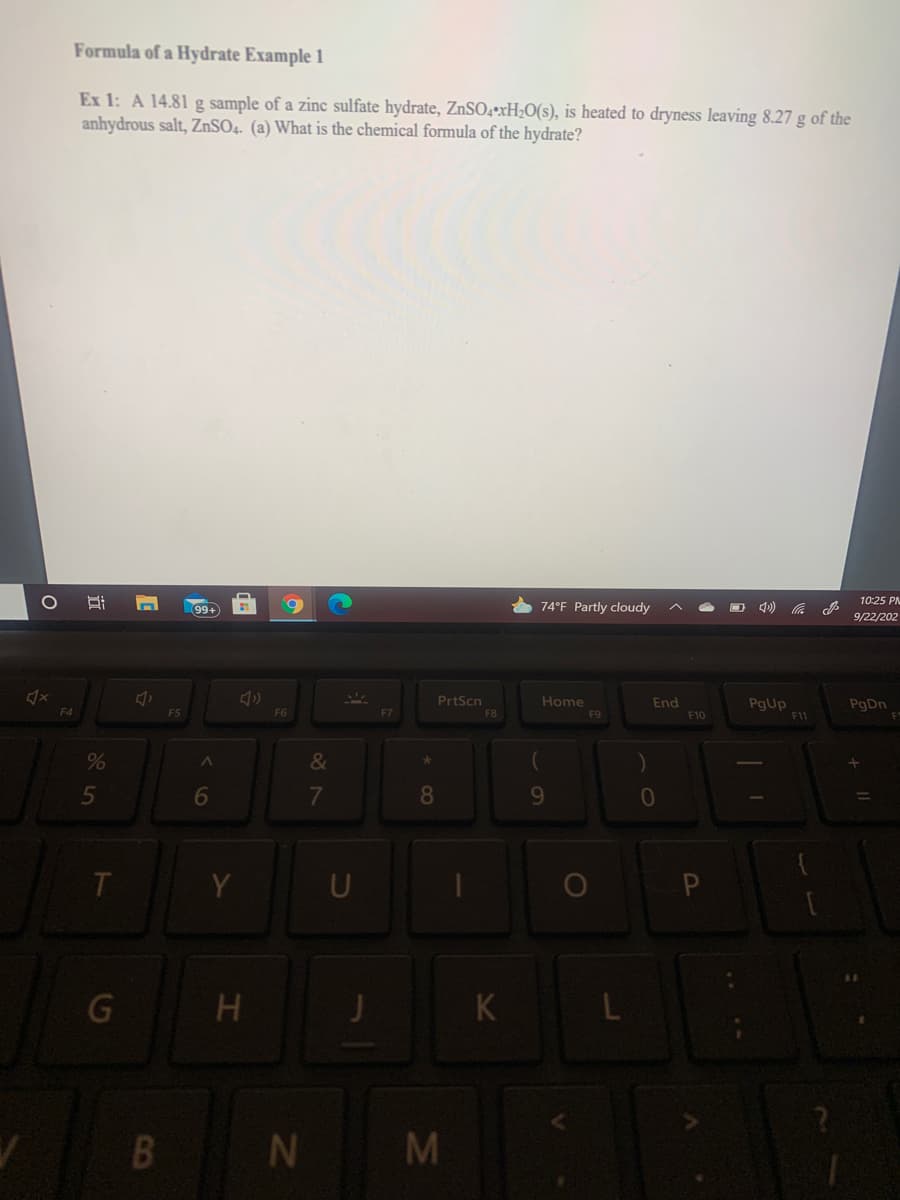
Transcribed Image Text:Formula of a Hydrate Example 1
Ex 1: A 14.81 g sample of a zinc sulfate hydrate, ZnSO4•xH;O(s), is heated to dryness leaving 8.27 g of the
anhydrous salt, ZnSO4. (a) What is the chemical formula of the hydrate?
10:25 PM
99+
74°F Partly cloudy
O 4) A
9/22/202
PrtScn
F8
Home
F9
End
PgUp
F4
F5
F6
F7
F10
F11
&
5
6
8
9-
U
G H
K L
L|N M
CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning