Caffeine occurs naturally in tea leaves and coffee beans. Pure caffeine (mp 236°C) is a white crystalline solid material at room temperature. It is classified as an alkaloid - a nitrogen-containing basic compound that is obtained from plants and has physiological effects in the body. A student pulverizes 8.78 grams (g) of coffee beans into a finely ground powder by using a mortar and pestle. The caffeine is then extracted into 300 mL of boiling dichloromethane (CH2CII2) by the same solid- liquid extraction technique used in Unit 1. After 30 minutes, the solution is filtered hot to remove a large amount of brown insoluble material. Dichloromethane is removed from the filtrate via rotary evaporation, leaving 916.3 milligrams (mg) of caffeine behind, as a white solid. What is the percentage of isolated caffeine with respect to the starting amount of crude coffee beans?
Caffeine occurs naturally in tea leaves and coffee beans. Pure caffeine (mp 236°C) is a white crystalline solid material at room temperature. It is classified as an alkaloid - a nitrogen-containing basic compound that is obtained from plants and has physiological effects in the body. A student pulverizes 8.78 grams (g) of coffee beans into a finely ground powder by using a mortar and pestle. The caffeine is then extracted into 300 mL of boiling dichloromethane (CH2CII2) by the same solid- liquid extraction technique used in Unit 1. After 30 minutes, the solution is filtered hot to remove a large amount of brown insoluble material. Dichloromethane is removed from the filtrate via rotary evaporation, leaving 916.3 milligrams (mg) of caffeine behind, as a white solid. What is the percentage of isolated caffeine with respect to the starting amount of crude coffee beans?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
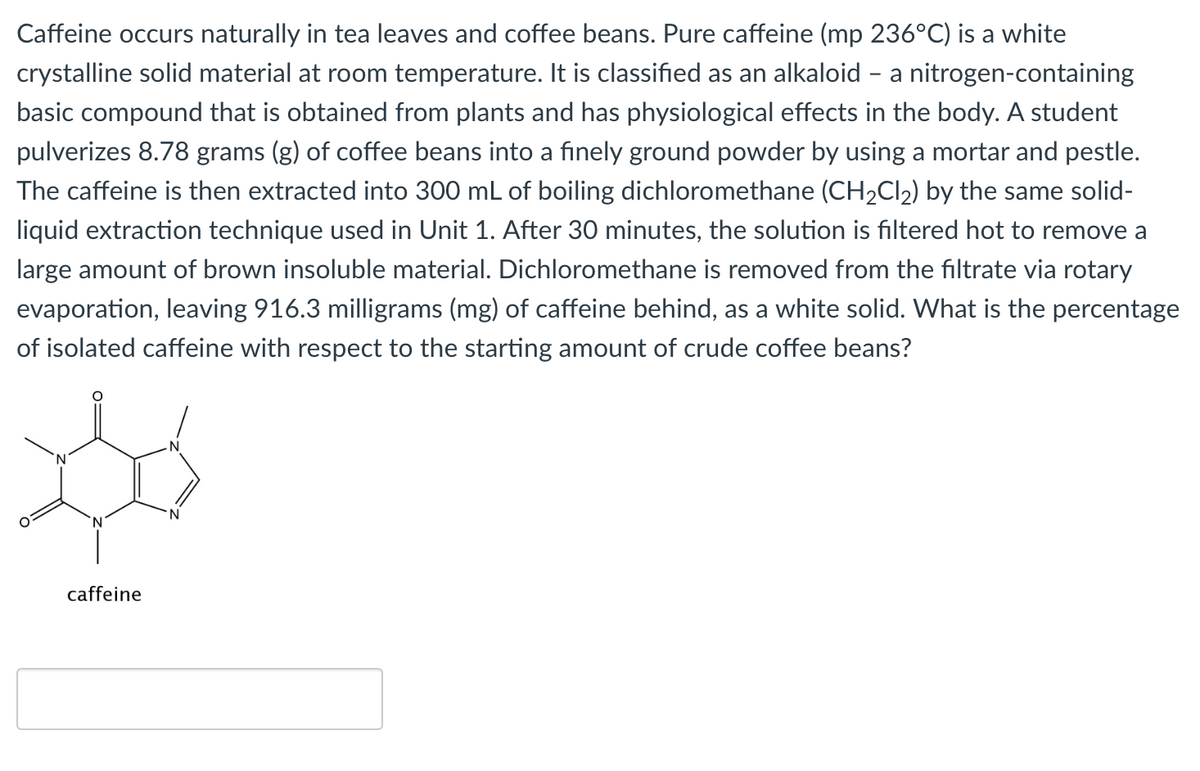
Transcribed Image Text:Caffeine occurs naturally in tea leaves and coffee beans. Pure caffeine (mp 236°C) is a white
crystalline solid material at room temperature. It is classified as an alkaloid - a nitrogen-containing
basic compound that is obtained from plants and has physiological effects in the body. A student
pulverizes 8.78 grams (g) of coffee beans into a finely ground powder by using a mortar and pestle.
The caffeine is then extracted into 300 mL of boiling dichloromethane (CH2CI2) by the same solid-
liquid extraction technique used in Unit 1. After 30 minutes, the solution is filtered hot to remove a
large amount of brown insoluble material. Dichloromethane is removed from the filtrate via rotary
evaporation, leaving 916.3 milligrams (mg) of caffeine behind, as a white solid. What is the percentage
of isolated caffeine with respect to the starting amount of crude coffee beans?
caffeine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning