Examine the diagram below 80 60 40 reactants 20 products 0 Reaction Progress c. What is the change in enthalpy with and without the presence of a catalysts? d. What role does the catalyst have in this reaction? Potential Energy (kJ) without catalyst with catalyst a. What is the energy of activation for the forward reaction with and without the catalyst? b. What is the energy of activation for the reverse reaction, with and without the presense of a catalyst ysts?
Examine the diagram below 80 60 40 reactants 20 products 0 Reaction Progress c. What is the change in enthalpy with and without the presence of a catalysts? d. What role does the catalyst have in this reaction? Potential Energy (kJ) without catalyst with catalyst a. What is the energy of activation for the forward reaction with and without the catalyst? b. What is the energy of activation for the reverse reaction, with and without the presense of a catalyst ysts?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.99PAE: Substances that poison a catalyst pose a major concern for many engineering designs, including those...
Related questions
Question
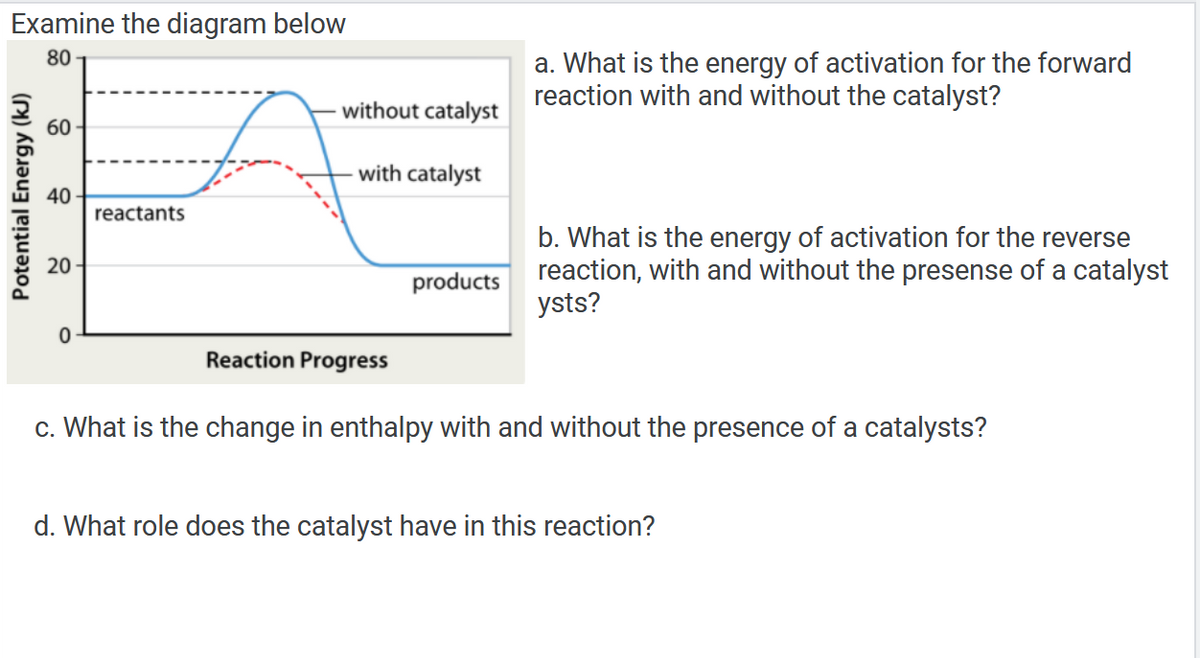
Transcribed Image Text:Examine the diagram below
80
60
40
reactants
20
products
0
Reaction Progress
c. What is the change in enthalpy with and without the presence of a catalysts?
d. What role does the catalyst have in this reaction?
Potential Energy (kJ)
without catalyst
with catalyst
a. What is the energy of activation for the forward
reaction with and without the catalyst?
b. What is the energy of activation for the reverse
reaction, with and without the presense of a catalyst
ysts?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning