Example 8.5, pg. 262 Ammonia, NH3, can be synthesized by this reaction: 2 NO(g) + 5 H2(g) 2 NH3(g) + 2 H,0(g) - What maximum amount of ammonia in grams can be synthesized from 45.8 g of NO and 12.4 g of H2?
Example 8.5, pg. 262 Ammonia, NH3, can be synthesized by this reaction: 2 NO(g) + 5 H2(g) 2 NH3(g) + 2 H,0(g) - What maximum amount of ammonia in grams can be synthesized from 45.8 g of NO and 12.4 g of H2?
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 35MP: One step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to...
Related questions
Question
Transcribed Image Text:3(2)
O Search
rida tariq
mations
Slide Show
Review
View
Help
21
AA A E
Shape Fill
Shape Outline v
Aav 2 A v
Shapes Arrange Quick
Styles
Shape Effects v
Paragraph
Drawing
Practice
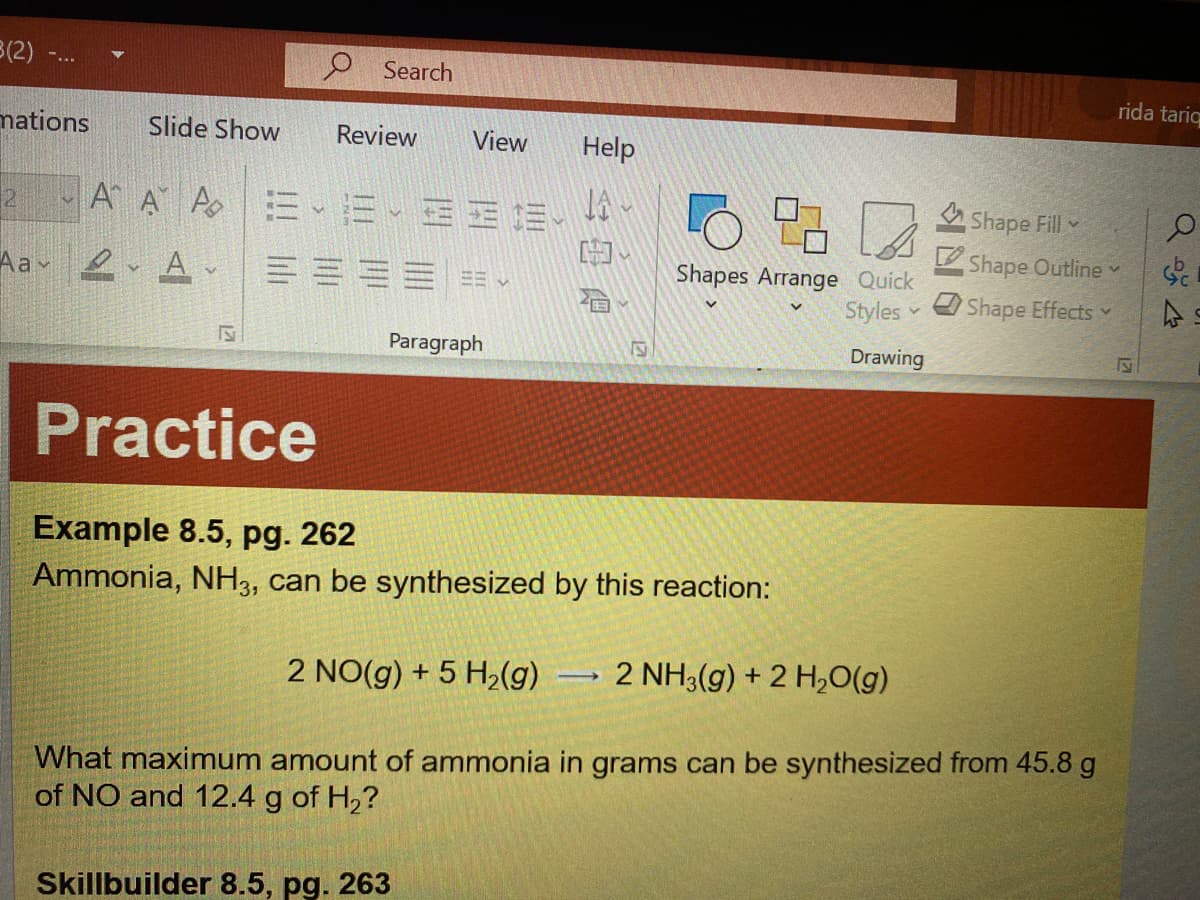
Example 8.5, pg. 262
Ammonia, NH3, can be synthesized by this reaction:
2 NO(g) + 5 H2(g)
2 NH3(g) + 2 H,0(g)
What maximum amount of ammonia in grams can be synthesized from 45.8 g
of NO and 12.4 g of H,?
Skillbuilder 8.5, pg. 263
12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning