9.) If 438g of Magnesium sulfate react with an excess of sodium chloride, how much magnesium chloride can be produced? MgSO4 + NACI --> MgCl2 + Na2SO4
9.) If 438g of Magnesium sulfate react with an excess of sodium chloride, how much magnesium chloride can be produced? MgSO4 + NACI --> MgCl2 + Na2SO4
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 33QAP: Dichlorine oxide is used as bactericide to purify water. It is produced by the chlorination of...
Related questions
Question
I need help
With number 9 and 10
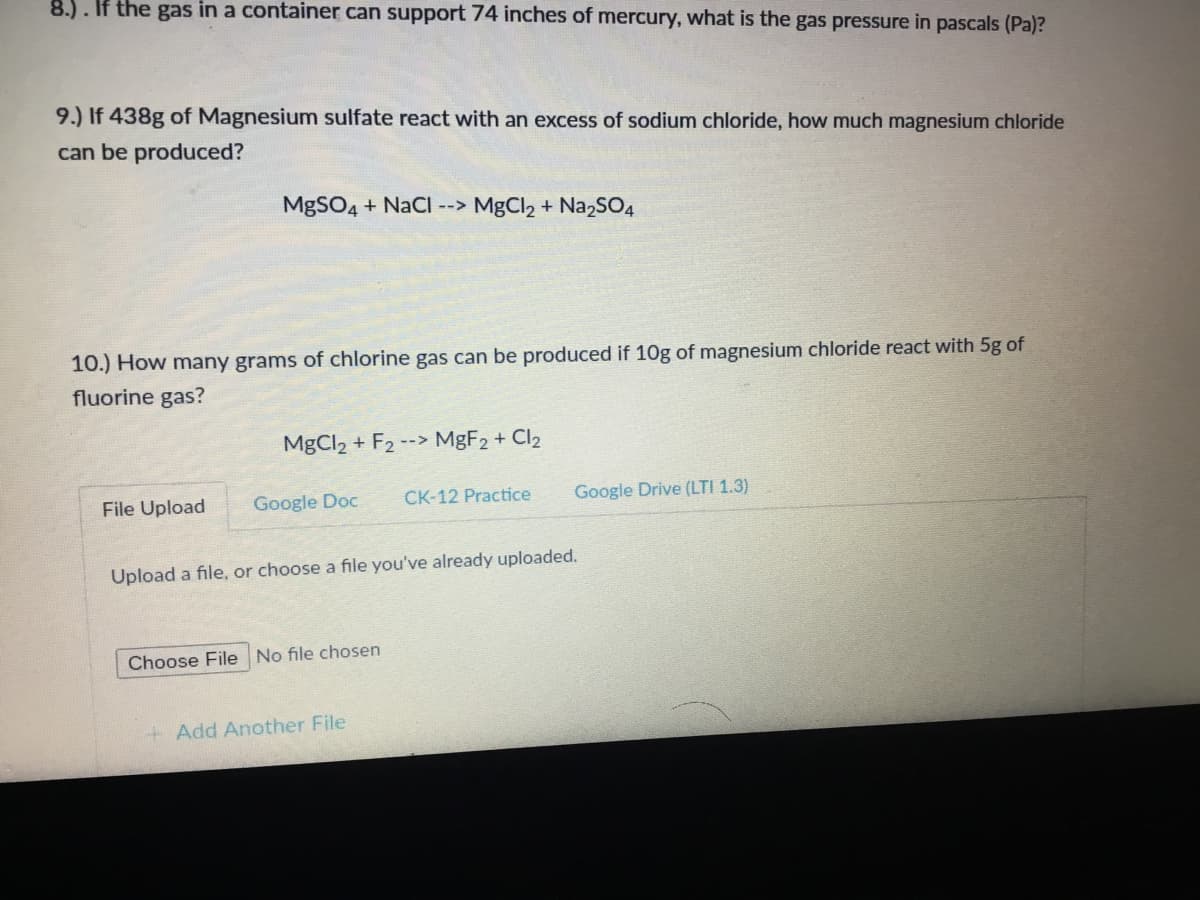
Transcribed Image Text:8.). If the gas in a container can support 74 inches of mercury, what is the gas pressure in pascals (Pa)?
9.) If 438g of Magnesium sulfate react with an excess of sodium chloride, how much magnesium chloride
can be produced?
MgSO4 + NaCI --> MgCl2 + Na2SO4
10.) How many grams of chlorine gas can be produced if 10g of magnesium chloride react with 5g of
fluorine gas?
MgCl2 + F2 --> MBF2 + Cl2
File Upload
Google Doc
CK-12 Practice
Google Drive (LTI 1.3)
Upload a file, or choose a file you've already uploaded.
Choose File No file chosen
+ Add Another File
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER