examples of Ssily the definitions in the introduction. Cr2 O3 + 2Al- 2Cr + Al½O3 CH4 + 202+ CO2 + 2H2O Mg + Cu?+→ Mg²+ + Cu Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help Magnesium in Mg + Cu2+Mg?+ + Cu Chromium in Aluminum in Cr,03 + 2A1→ 2Cr + Al2O3 Cr2O3 +2Al 2Cr + Al203 Oxygen in CH4 + 202- CO2 + 2H20 Copper in Carbon in Mg + Cu2+Mg²+ + Cu CH4 +202 CO2 + 2H20 Element is oxidized Element is reduced
examples of Ssily the definitions in the introduction. Cr2 O3 + 2Al- 2Cr + Al½O3 CH4 + 202+ CO2 + 2H2O Mg + Cu?+→ Mg²+ + Cu Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help Magnesium in Mg + Cu2+Mg?+ + Cu Chromium in Aluminum in Cr,03 + 2A1→ 2Cr + Al2O3 Cr2O3 +2Al 2Cr + Al203 Oxygen in CH4 + 202- CO2 + 2H20 Copper in Carbon in Mg + Cu2+Mg²+ + Cu CH4 +202 CO2 + 2H20 Element is oxidized Element is reduced
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter8: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 3STP
Related questions
Question
100%
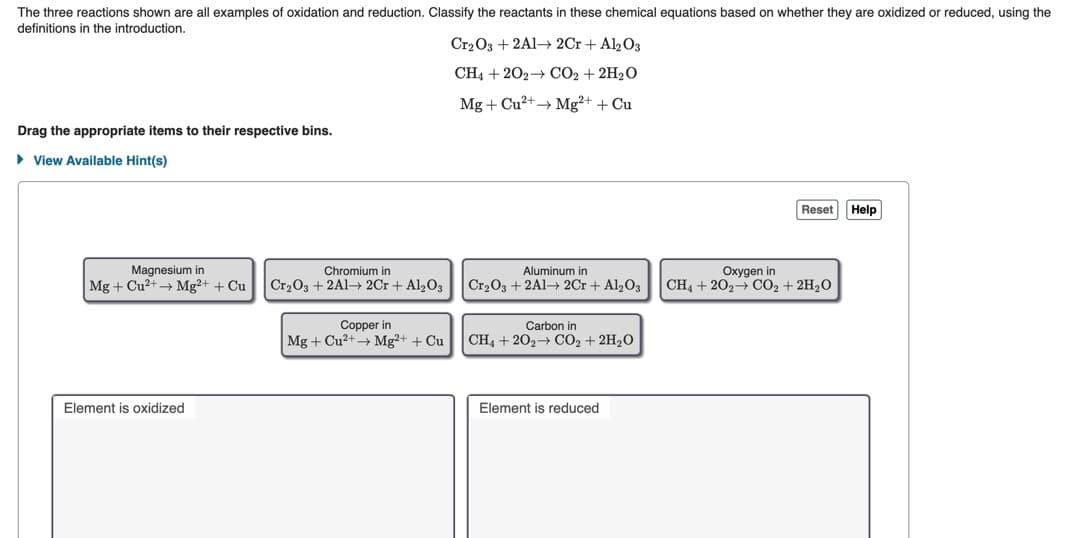
Transcribed Image Text:The three reactions shown are all examples of oxidation and reduction. Classify the reactants in these chemical equations based on whether they are oxidized or reduced, using the
definitions in the introduction.
Cr2 O3 + 2A1→ 2Cr + Al2O3
CH4 + 202→ CO2 + 2H2O
Mg + Cu2+→ Mg²+ + Cu
Drag the appropriate items to their respective bins.
• View Available Hint(s)
Reset Help
Magnesium in
Mg + Cu?+→ Mg²+ + Cu
Chromium in
Aluminum in
Oxygen in
CH4 + 202→ CO2 + 2H2O
Cr2O3 + 2A1→ 2Cr + Al2O3
Cr203 + 2A1→ 2Cr + Al,03
Copper in
Mg + Cu2+ Mg²+ + Cu
Carbon in
CH4 + 202 CO2 + 2H2O
Element is oxidized
Element is reduced
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning