Exercise 17.117 Part A In analytical chemistry, bases used for titrations must often be standardized; that is, their concentration must be precisely determined. Standardization of sodium hydroxide solutions is often accomplished by titrating potassium hydrogen phthalate (KHC3H4O4), also know as KHP, with the NaOH solution to be standardized. Write an equation for the reaction between NAOH and KHP. O NAOH + KHC3H4O4 → Nat + K+ + HC8H40 + OH O NAOH + 2 KHC&H4O4 → Na+ +K* + 2C3H4O? +2 H2O O NAOH + 2 KHC3H4O4 → Na+ +K* +2HC3H4O? +20H O NAOH + KHC3H4O4 → Na* + K* + C3H4O + H2O
Exercise 17.117 Part A In analytical chemistry, bases used for titrations must often be standardized; that is, their concentration must be precisely determined. Standardization of sodium hydroxide solutions is often accomplished by titrating potassium hydrogen phthalate (KHC3H4O4), also know as KHP, with the NaOH solution to be standardized. Write an equation for the reaction between NAOH and KHP. O NAOH + KHC3H4O4 → Nat + K+ + HC8H40 + OH O NAOH + 2 KHC&H4O4 → Na+ +K* + 2C3H4O? +2 H2O O NAOH + 2 KHC3H4O4 → Na+ +K* +2HC3H4O? +20H O NAOH + KHC3H4O4 → Na* + K* + C3H4O + H2O
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.16QAP
Related questions
Question
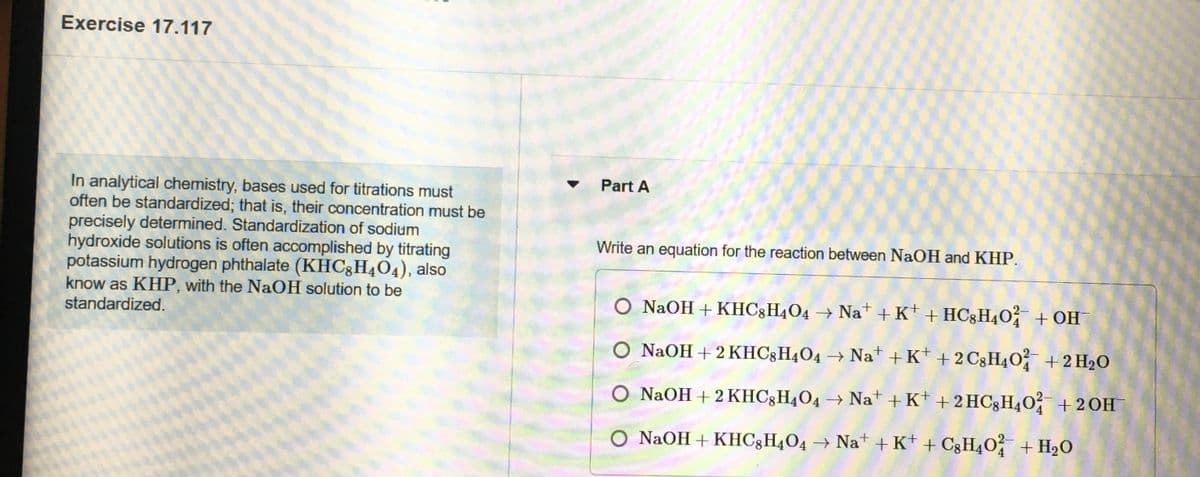
Transcribed Image Text:Exercise 17.117
Part A
In analytical chemistry, bases used for titrations must
often be standardized; that is, their concentration must be
precisely determined. Standardization of sodium
hydroxide solutions is often accomplished by titrating
potassium hydrogen phthalate (KHC3H4O4), also
know as KHP, with the NaOH solution to be
standardized.
Write an equation for the reaction between NaOH and KHP.
O NAOH+KHC;H4O4 → Nat + K* + HC§H4O² + OH¯
O NAOH + 2 KHC3H4O4 → Na* + K* + 2 C3H4O +2 H2O
O NAOH + 2KHC3H4O4 → Nat + K+ + 2 HC3H40 +2OH
O NAOH + KHC3H4O4 → Na* + K+ + C3H4O + H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

