Exp. No. 4 5 6 Volume of Acetic acid Volume of Water Mass of beaker and liquid Mass of Alka Total mass of beaker and Seltzer tablet contents before reaction 15 my 20 m² 158. 080g 3.240g me 20m 25 10ML 157.401g 3.244g Total mass of beaker and contents after reaction 15 m 145.477g |3.228g|168.7059 160.663g Mass of CO2 (loss of mass) 161.2769 160.345g 0.931g 167.850g 0.855 159.6719 0.982 Mass of NaHCO3 in tablet* % of NaHCO3 in tablet
Exp. No. 4 5 6 Volume of Acetic acid Volume of Water Mass of beaker and liquid Mass of Alka Total mass of beaker and Seltzer tablet contents before reaction 15 my 20 m² 158. 080g 3.240g me 20m 25 10ML 157.401g 3.244g Total mass of beaker and contents after reaction 15 m 145.477g |3.228g|168.7059 160.663g Mass of CO2 (loss of mass) 161.2769 160.345g 0.931g 167.850g 0.855 159.6719 0.982 Mass of NaHCO3 in tablet* % of NaHCO3 in tablet
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.13QAP
Related questions
Question
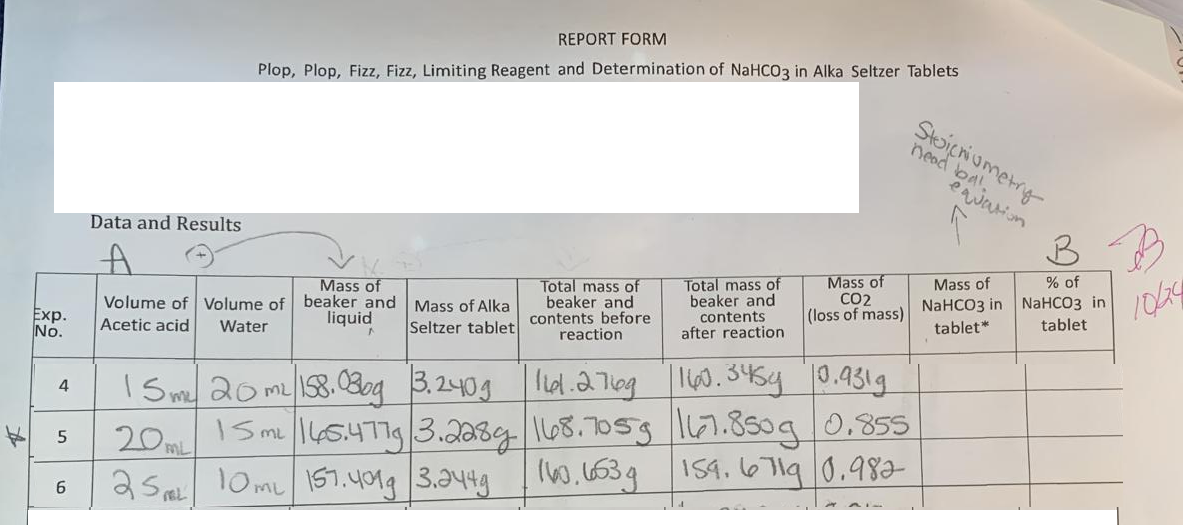
Transcribed Image Text:Exp.
No.
4
5
6
Data and Results
A
REPORT FORM
Plop, Plop, Fizz, Fizz, Limiting Reagent and Determination of NaHCO3 in Alka Seltzer Tablets
Mass of
Volume of Volume of beaker and
liquid
Water
Acetic acid
Mass of Alka
Seltzer tablet
15 m² 20 m² 158. 030g 3.240g
me
20ML
25 10ML 157.401g 3.244g
FEL
Total mass of
beaker and
contents before
reaction
Total mass of
beaker and
contents
after reaction
Mass of
CO2
(loss of mass)
161.2769 160.345g 10.931g
15 m 145.477g 3.2289 168.7059 167.850g 0.855
160.653g 159.6719 0.982
Stoichiometry
nead bal.
equation
B
Mass of
% of
NaHCO3 in. NaHCO3 in
tablet*
tablet
10/24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

