Experiment 3 Repeat the experiment above (Question 2) using a 10 mL pipette to dispense the 10 mL of water. RESULTS- Table #. 10 mL pipette calibration raw data (in g) Replicates 3 4 5 Initial beaker weight (g) 13.1499 Total weight (g) Weight of water dispensed (g) Mean weight of water dispensed 23.1284 33.1156 43.1149 53.0098 63.0087 SD RSD Mean volume of water dispensed Calculate: a) The mean weight, standard deviation and RSD of the water dispensed. b) The actual volume dispensed using the water density table (APPENDIX) and the temperature provided..
Experiment 3 Repeat the experiment above (Question 2) using a 10 mL pipette to dispense the 10 mL of water. RESULTS- Table #. 10 mL pipette calibration raw data (in g) Replicates 3 4 5 Initial beaker weight (g) 13.1499 Total weight (g) Weight of water dispensed (g) Mean weight of water dispensed 23.1284 33.1156 43.1149 53.0098 63.0087 SD RSD Mean volume of water dispensed Calculate: a) The mean weight, standard deviation and RSD of the water dispensed. b) The actual volume dispensed using the water density table (APPENDIX) and the temperature provided..
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.12QAP
Related questions
Question
3a
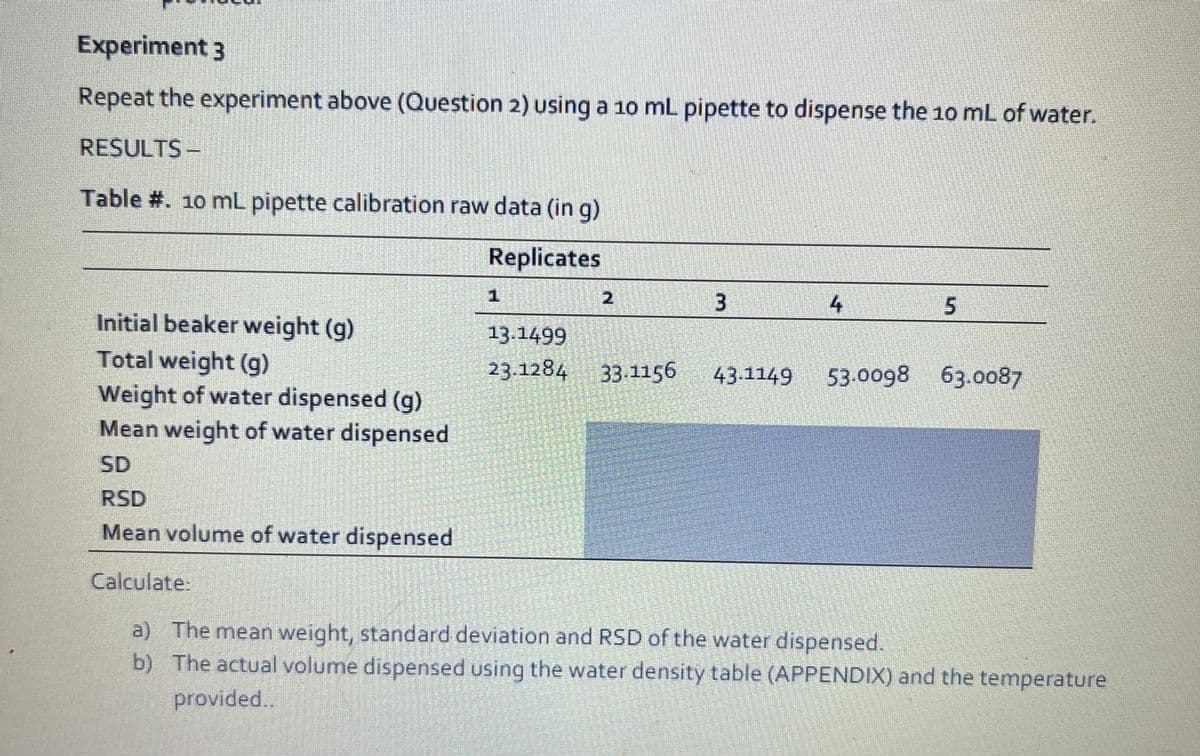
Transcribed Image Text:Experiment 3
Repeat the experiment above (Question 2) using a 10 mL pipette to dispense the 10 mL of water.
RESULTS -
Table #. 10 mL pipette calibration raw data (in g)
Replicates
1
2
Initial beaker weight (g)
Total weight (g)
Weight of water dispensed (g)
Mean weight of water dispensed
13.1499
23.1284 33-1156
43-1149
53.0098 63.0087
SD
RSD
Mean volume of water dispensed
Calculate.
a) The mean weight, standard deviation and RSD of the water dispensed.
b) The actual volume dispensed using the water density table (APPENDIX) and the temperature
provided..
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning