lame odium chloride (example) otassium bromide Cation Anion Formula Na* CI NaCl Ca2+ 02- SrCl2 agnesium sulfide Cs3N sium oxide Al3+ F1- uminum sulfide Lit+ 02- rontium nitride Ca2+ p3- Al203
lame odium chloride (example) otassium bromide Cation Anion Formula Na* CI NaCl Ca2+ 02- SrCl2 agnesium sulfide Cs3N sium oxide Al3+ F1- uminum sulfide Lit+ 02- rontium nitride Ca2+ p3- Al203
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 29E
Related questions
Question
100%
I have already completed the lab as a study guide but I want to compare my data!
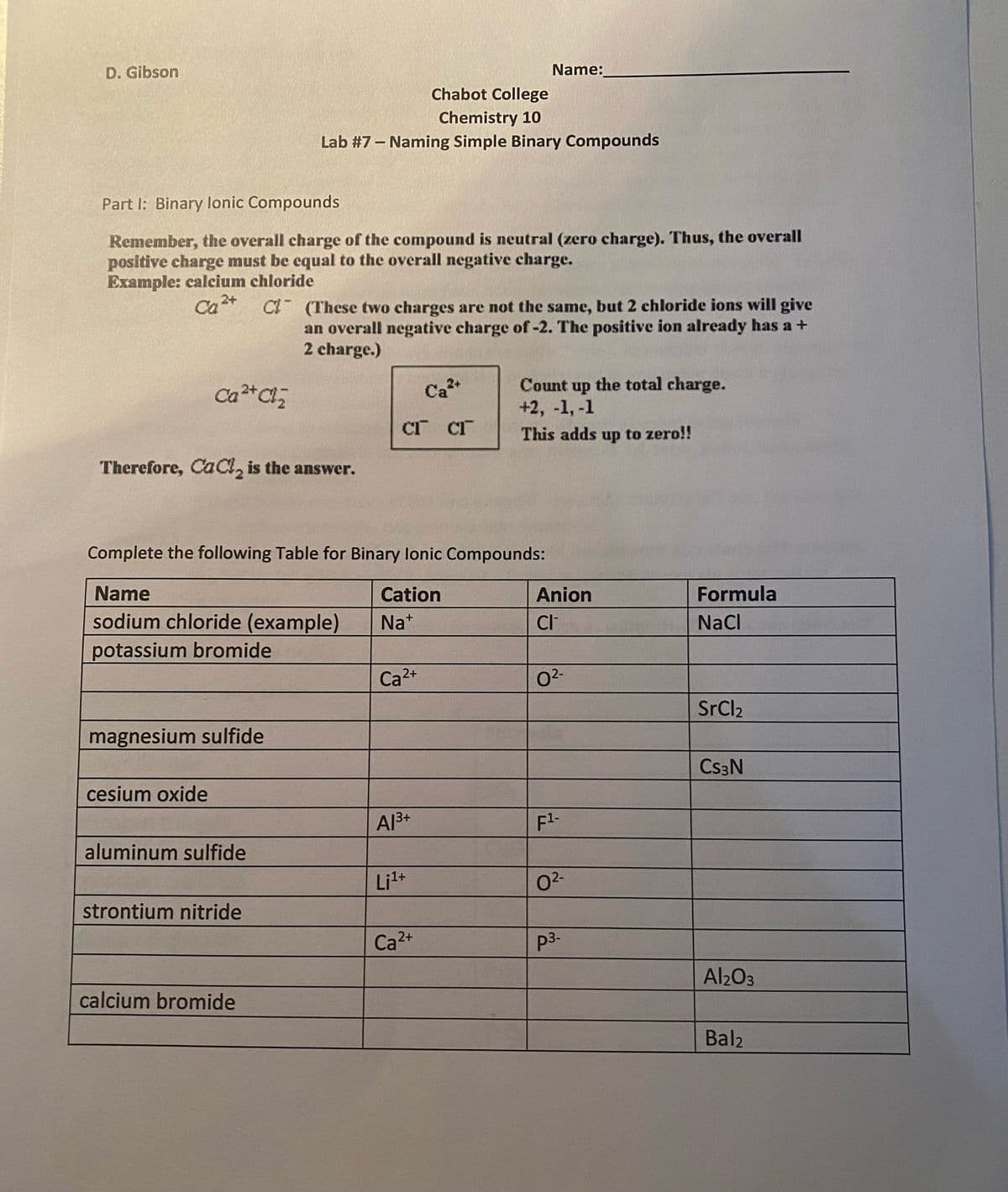
Transcribed Image Text:D. Gibson
Name:
Chabot College
Chemistry 10
Lab #7 – Naming Simple Binary Compounds
Part I: Binary lonic Compounds
Remember, the overall charge of the compound is neutral (zero charge). Thus, the overall
positive charge must be equal to the overall negative charge.
Example: calcium chloride
CI (These two charges are not the same, but 2 chloride ions will give
an overall negative charge of -2. The positive ion already has a +
2 charge.)
Ca
2+
Count
up
the total charge.
+2, -1, -1
CT CI
This adds up to zero!!
Therefore, CaCl, is the answer.
Complete the following Table for Binary lonic Compounds:
Name
Cation
Anion
Formula
sodium chloride (example)
Na*
CI
NaCl
potassium bromide
Ca2+
02-
SrCl2
magnesium sulfide
Cs3N
cesium oxide
Al3+
F1-
aluminum sulfide
:1+
02-
strontium nitride
Ca2+
p3-
Al2O3
calcium bromide
Bal2

Transcribed Image Text:Part II: Binary Covalent Compounds
Covalent compounds are produces by nonmetals bonding with one another. You can use the valence
electrons for each element to determine the simplest formula that exists between two elements. For
example, H has 1 valence electron and needs one more to obey the Octet Rule, so it will only make 1
bond when found in a compound. Oxygen has 6 valence electrons and needs two more to obey the
Octet Rule, so it typically makes 2 bonds when found in a compound. Since hydrogen needs just one
electron while oxygen needs two, the correct formula for the simplest compound would be H20.
However, there are often other combinations possible in addition to the simplest formula. For
example, hydrogen and oxygen can also form, H2O2, which is known as hydrogen peroxide. To work
with all the possible combinations, some rules have been developed for naming binary covalent
compounds. The rules are stated below:
1. The least metallic element will always end in the suffix "-ide". Remember that as you move to
the right in a period, the elements become less metallic and as you move down a group, the
elements become more metallic.
2. Prefixes will be used to indicate the number of atoms of each element in the formula.
a. The first ten prefixes in increasing order are: mono, di, tri, tetra, penta, hexa, hepta,
octa, nona and deca.
b. If there is only one atom of the first element, the prefix mono is left out. For example,
CO is carbon monoxide, not monocarbon monoxide.
C. If a prefix ends in an "a" or "o" and the name of the element also starts with a vowel,
the "a" or "o" of the prefix is dropped. Example, carbon monoxide - not carbon
monooxide.
3. Some compounds still go by their common names – such as water (H2O), ammonia (NH3) and
hydrogen peroxide (H2O2).
Complete the following table:
Name
Formula
sulfur dioxide
SO3
nitrogen trioxide
Cl207
tricarbon disulfide
SCI2
disulfur dichloride
P2S5
Silicon dioxide
P4010
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning