EXPERIMENT 6 PRE-LABORATORY QUESTIONS DETERMINING THE SOLUBILITY OF AN UNKNOWN IONIC COMPOUND NAME DATE 1. Caleulate the concentration, in grams of solute per 100 g of water, of a solution in which 7.462 g of solute dissolved in 25.50 mlL of water. 2 Sketch two graphs, the first for a solid that's solubility is increasing as a function of temperature, and the second for a solid whose solubility decreases as a funetion of temperature. Be sure to label each axis. a) b) 3. After the first 7.00 ml, how many mlL of distilled water are added to the test tube for each subsequent measurement? 4. How many decimal values can you measure with a buret? EXPERIMENT 6 107
EXPERIMENT 6 PRE-LABORATORY QUESTIONS DETERMINING THE SOLUBILITY OF AN UNKNOWN IONIC COMPOUND NAME DATE 1. Caleulate the concentration, in grams of solute per 100 g of water, of a solution in which 7.462 g of solute dissolved in 25.50 mlL of water. 2 Sketch two graphs, the first for a solid that's solubility is increasing as a function of temperature, and the second for a solid whose solubility decreases as a funetion of temperature. Be sure to label each axis. a) b) 3. After the first 7.00 ml, how many mlL of distilled water are added to the test tube for each subsequent measurement? 4. How many decimal values can you measure with a buret? EXPERIMENT 6 107
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
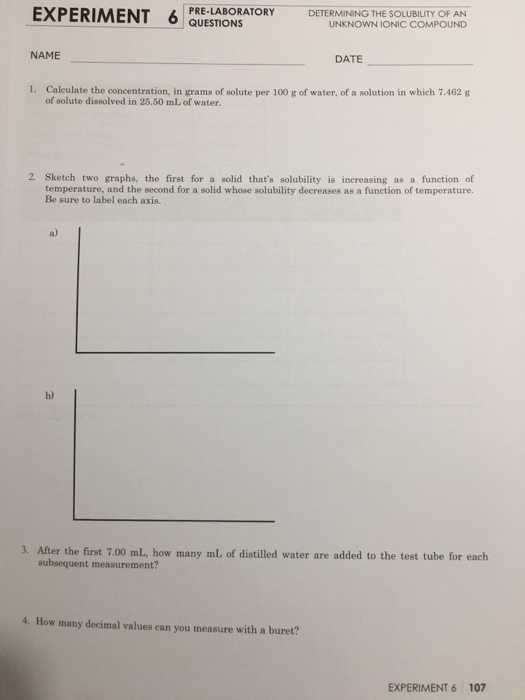
Transcribed Image Text:EXPERIMENT 6
PRE-LABORATORY
QUESTIONS
DETERMINING THE SOLUBILITY OF AN
UNKNOWN IONIC COMPOUND
NAME
DATE
1. Calculate the concentration, in grams of solute per 100 g of water, of a solution in which 7.462 g
of solute dissolved in 25.50 mL of water.
2. Sketch two graphs, the first for a solid that's solubility is increasing as a funetion of
temperature, and the second for a solid whose solubility decreases as a funetion of temperature.
Be sure to label each axis.
b)
3. After the first 7.00 mL, how many mL of distilled water are added to the test tube for each
subsequent measurement?
4. How many decimal values can you measure with a buret?
EXPERIMENT 6 107
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT