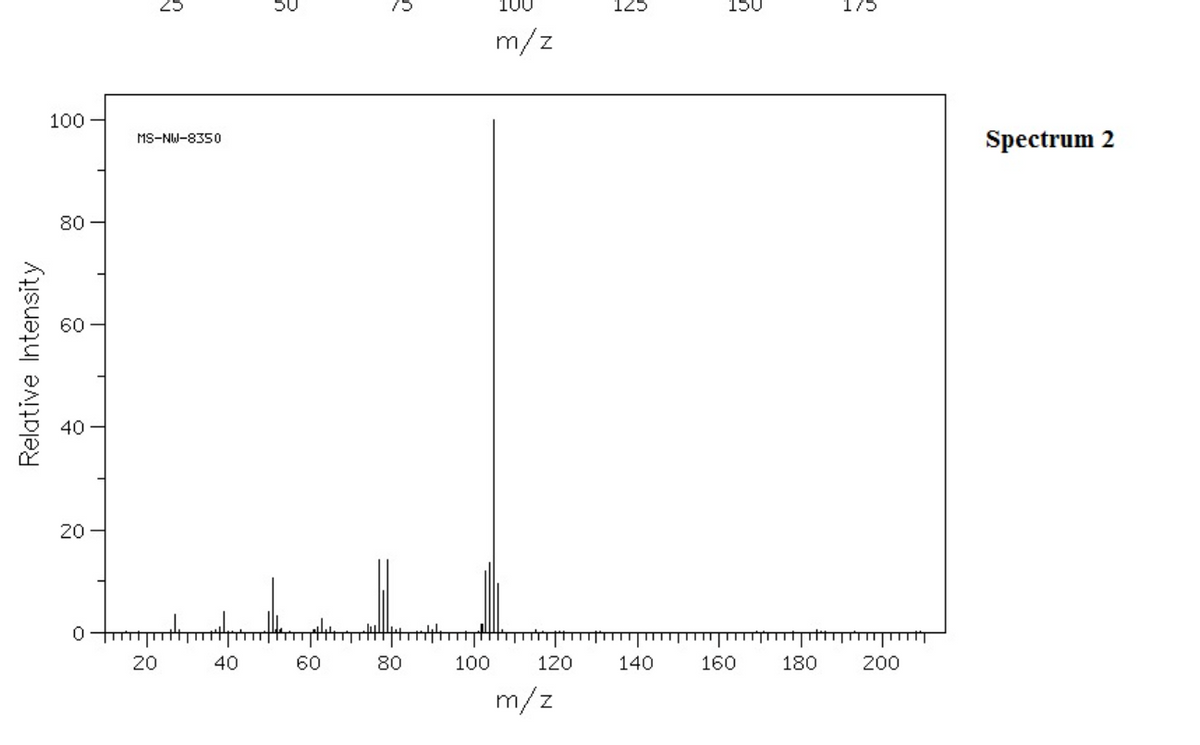
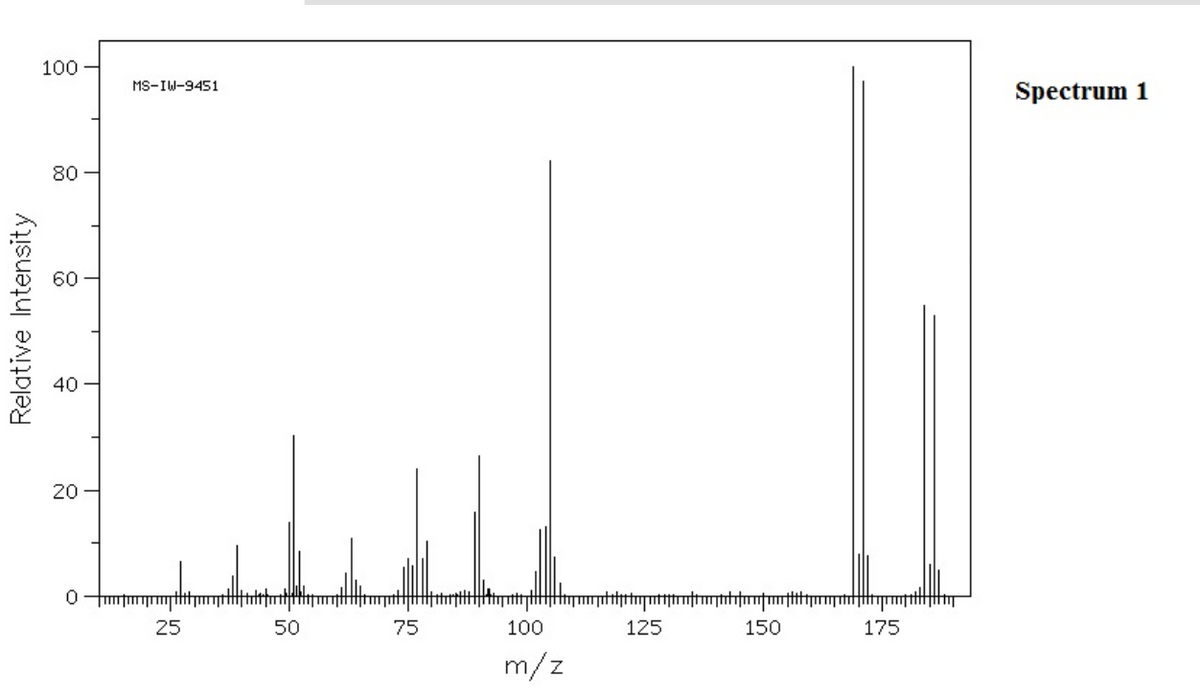
EXPLAIN differences in fragmentation in isomers The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene, respectively.
EXPLAIN differences in fragmentation in isomers The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene, respectively.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 77PS
Related questions
Question
EXPLAIN differences in fragmentation in isomers
The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene, respectively.
Transcribed Image Text:75
100
125
150
175
m/z
100
Spectrum 2
MS-NW-8350
80
60
40
20
20
40
60
80
100
120
140
160
180
200
m/z
Relative Intensity
Transcribed Image Text:100
MS-IW-9451
Spectrum 1
80
20
25
75
100
125
150
175
m/z
50
우
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning