Explain in detail how you would separate benozic acid from phenol, using either lithium hydroxide, sodium bicarbonate, and any acid? Specify which compound would be what layer whenever a reagent is added. Please indicate how you would recover the native form of any compound that was converted during extraction. LOH OH Phenol Benozic acid pka =~4.0 pka = 10
Explain in detail how you would separate benozic acid from phenol, using either lithium hydroxide, sodium bicarbonate, and any acid? Specify which compound would be what layer whenever a reagent is added. Please indicate how you would recover the native form of any compound that was converted during extraction. LOH OH Phenol Benozic acid pka =~4.0 pka = 10
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter16: The Sn2 Reaction: 1-bromobutane
Section: Chapter Questions
Problem 2Q
Related questions
Question
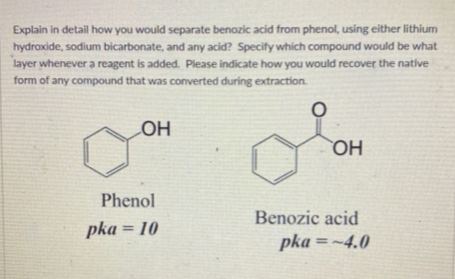
Transcribed Image Text:Explain in detail how you would separate benozic acid from phenol, using either lithium
hydroxide, sodium bicarbonate, and any acid? Specify which compound would be what
layer whenever a reagent is added. Please indicate how you would recover the native
form of any compound that was converted during extraction.
LOH
OH
Phenol
Benozic acid
pka = 10
pka =~4.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole