Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 66E
Related questions
Question
100%
Please help me answer the questions
Transcribed Image Text:1ons Reviewed -QU
O Presentation Session Student
A app.peardeck.com/student/taszvbbad?gooleClassroom-true
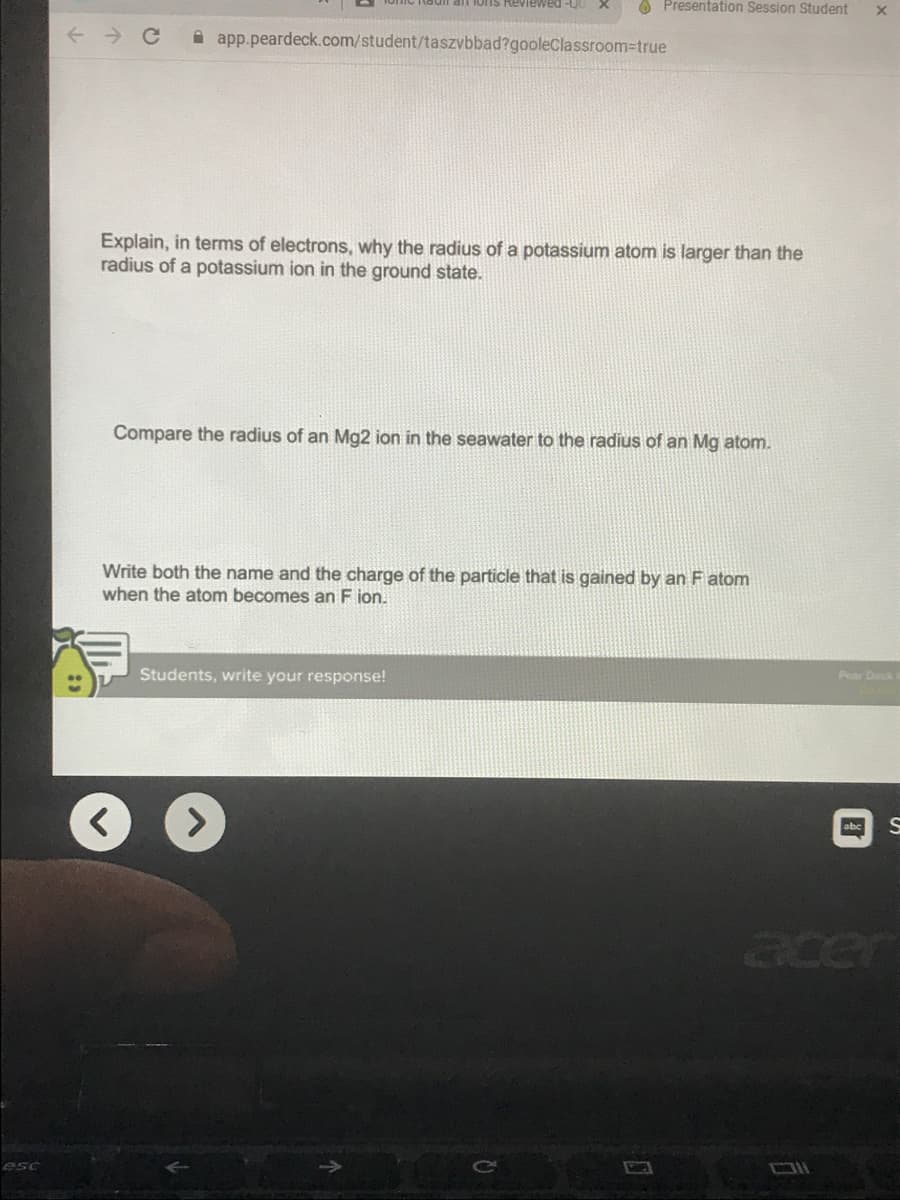
Explain, in terms of electrons, why the radius of a potassium atom is larger than the
radius of a potassium ion in the ground state.
Compare the radius of an Mg2 ion in the seawater to the radius of an Mg atom.
Write both the name and the charge of the particle that is gained by an F atom
when the atom becomes an F ion.
Students, write your response!
Pear Deck
abe S
acer
esc
C\
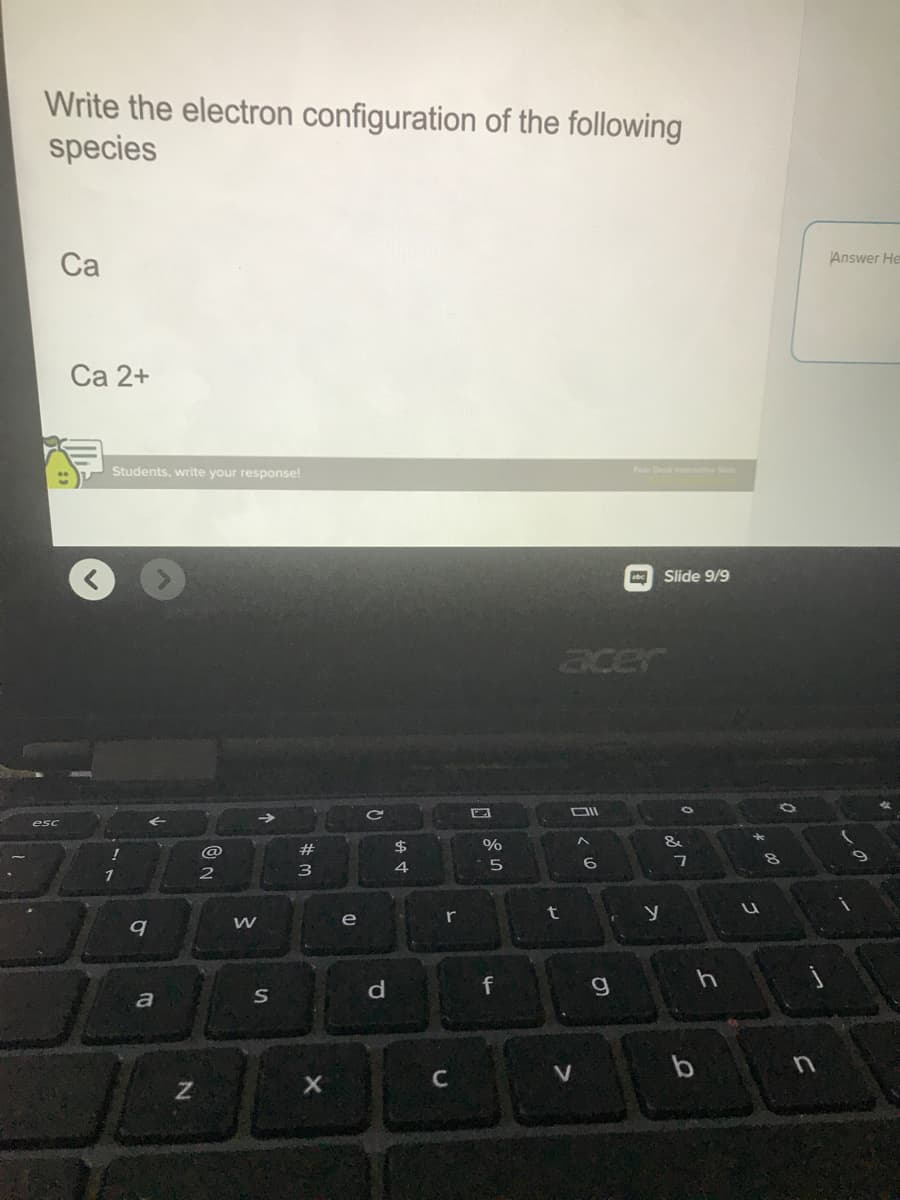
Transcribed Image Text:Write the electron configuration of the following
species
Ca
Answer He
Ca 2+
Students, write your response!
Pear Deck ineive
Slide 9/9
acer
DII
esc
$
%
&
#
4
6.
2
e
r
t
f
g.
b
C
ーの
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning