Explain the effect of each of the following stresses on the position of the equilibrium SO;(g) 2 SO02(g) + 2 O2(g) The reaction as written is endothermic. (a) O2(g) is added to the equilibrium mixture without changing volume or temperature. (b) The mixture is compressed at constant temperature. (c) The equilibrium mixture is cooled. (d) An inert gas is pumped into the equilibrium mixture while the total gas pressure and the temperature are kept constant. (e) An inert gas is added to the equilibrium mixture with- out changing the volume.
Explain the effect of each of the following stresses on the position of the equilibrium SO;(g) 2 SO02(g) + 2 O2(g) The reaction as written is endothermic. (a) O2(g) is added to the equilibrium mixture without changing volume or temperature. (b) The mixture is compressed at constant temperature. (c) The equilibrium mixture is cooled. (d) An inert gas is pumped into the equilibrium mixture while the total gas pressure and the temperature are kept constant. (e) An inert gas is added to the equilibrium mixture with- out changing the volume.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question
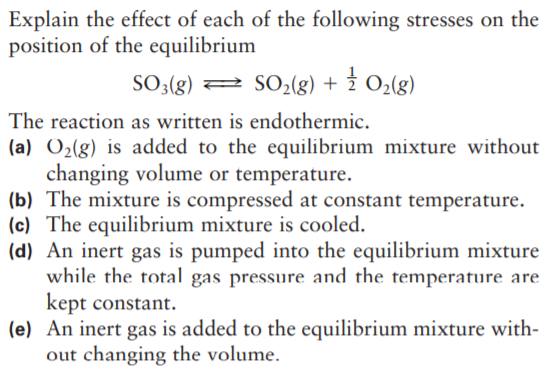
Transcribed Image Text:Explain the effect of each of the following stresses on the
position of the equilibrium
SO;(g)
2 SO02(g) + 2 O2(g)
The reaction as written is endothermic.
(a) O2(g) is added to the equilibrium mixture without
changing volume or temperature.
(b) The mixture is compressed at constant temperature.
(c) The equilibrium mixture is cooled.
(d) An inert gas is pumped into the equilibrium mixture
while the total gas pressure and the temperature are
kept constant.
(e) An inert gas is added to the equilibrium mixture with-
out changing the volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
