Explain the Titrimetric profile of Lysine in figure 2
Chapter1: Ready, Set, Go
Section: Chapter Questions
Problem 1S
Related questions
Question
Explain the Titrimetric profile of Lysine in figure 2.
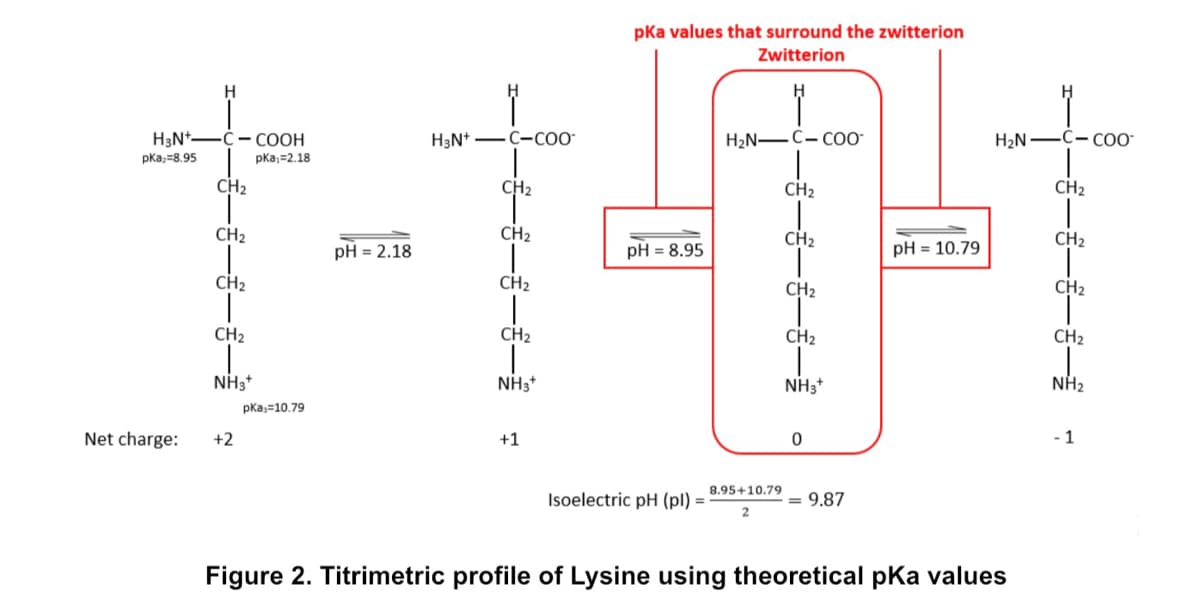
Transcribed Image Text:pka values that surround the zwitterion
Zwitterion
H.
H3N*-Ć -COOH
H3N* -C-CO"
H2N–C- C0-
H2N-C-CO-
pka,=8.95
pka;=2.18
CH2
CH2
CH2
CH2
CH2
CH2
CH2
CH2
pH = 2.18
pH = 8.95
pH = 10.79
CH2
CH2
CH2
CH2
CH2
CH2
CH2
CH2
NH3+
NH3*
NH3*
NH2
pKa=10.79
Net charge:
+2
+1
- 1
8.95+10.79
Isoelectric pH (pl) =
= 9.87
Figure 2. Titrimetric profile of Lysine using theoretical pKa values
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage



Essentials of Pharmacology for Health Professions
Nursing
ISBN:
9781305441620
Author:
WOODROW
Publisher:
Cengage

