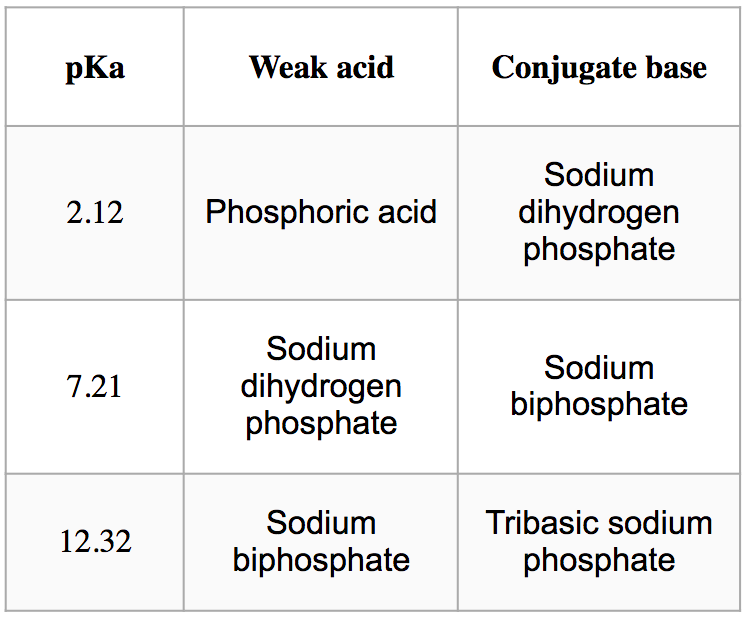
Extraction of DNA involves the use of a 1 M phosphate buffer (1-liter volume) having the hydrogen ion concentration of 6.165950019 x 10-9 M. Three pKa of phosphoric acid are available Concentrated phosphoric acid (85% w/w; Sp. Gr. = 1.70; MW = 98 g/mol) Sodium dihydrogen phosphate monohydrate (MW = 138g/mol) Sodium biphosphate heptahydrate (MW = 268g/mol) Tribasic sodium phosphate monohydrate (MW = 182 g/mol) Sodium hydroxide pellets (40 grams/mol) Question: What is the pH of the solution? (answer in two decimal places) How much of the weak acid component should be used? (Note for answer: Round to the nearest hundredth and include the unit such as "grams or milliliters"; Ex. 100.00 grams or 100.00g for weight & 100.00 milliliters or 100.00 mL for volume) Question: What is the suitable buffer component to be used? (Note for the answer: Separate the two components with “and”; Ex: Ammonia and Ammonium chloride) Question: How much of the salt component should be used? Question: Which component is greater in terms of molar ratio? (weak acid or salt)
Extraction of DNA involves the use of a 1 M phosphate buffer (1-liter volume) having the hydrogen ion concentration of 6.165950019 x 10-9 M. Three pKa of phosphoric acid are available
Concentrated phosphoric acid (85% w/w; Sp. Gr. = 1.70; MW = 98 g/mol)
Sodium dihydrogen phosphate monohydrate (MW = 138g/mol)
Sodium biphosphate heptahydrate (MW = 268g/mol)
Tribasic sodium phosphate monohydrate (MW = 182 g/mol)
Sodium hydroxide pellets (40 grams/mol)
Question: What is the pH of the solution? (answer in two decimal places)
How much of the weak acid component should be used?
(Note for answer: Round to the nearest hundredth and include the unit such as "grams or milliliters"; Ex. 100.00 grams or 100.00g for weight & 100.00 milliliters or 100.00 mL for volume)
Question: What is the suitable buffer component to be used?
(Note for the answer: Separate the two components with “and”; Ex: Ammonia and Ammonium chloride)
Question: How much of the salt component should be used?
Question: Which component is greater in terms of molar ratio? (weak acid or salt)
Step by step
Solved in 4 steps









