Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.3PAE
Related questions
Question
100%
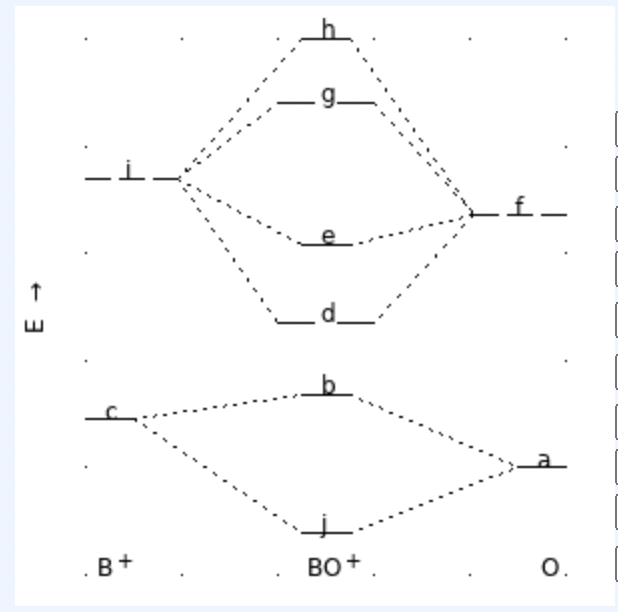
Given that the following MO-energy-level diagram applies to the the diatomic molecular cation BO+, match the letter label of each energy level with the corresponding orbital descriptor. (See attached image!)
O2p
σ2p
σ2s*
π2p
σ2p*
B+2s
π2p*
σ2s
O2s
B+2p
Does this diagram display the effect of s-p orbital mixing? YES or NO?
Which kind of magnetism would be observed for this cation?
paramagnetism
diamagnetism
What is the bond order for this cation? 0.0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 2.0
Transcribed Image Text:f.
a
B+
BO+
O.
+ 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning