Fe*(aq) + KSCN(s) →FESCN²+(aq) + K*(aq) To determine the moles of Fe3*(aq) in a 100.0 mL sample of an unknown solution, excess KSCN(s) is added to convert all the Fe3*(aq) species into the dark red species FeSCN²*(aq), as represented by the equation above. The absorbance of FESCN2*(aq) at different concentrations is shown in the graph below. 0.50 0.40 0.30 0.20 0.10 5x 10-5 10 x 10-5 Concentration of FeSCN²* (M) If the absorbance of the mixture is 0.20 at 453 nm, how many moles of Fe3+(aq) were present in the 100.0 mL sample? (Assume that any volume change due to adding KSCN is negligible.) О Зх 106 mol O 4x 104 mol 4x 10-6 mol O 2x 105 mol О 3х 104 mol Absorbance at 453 nm
Fe*(aq) + KSCN(s) →FESCN²+(aq) + K*(aq) To determine the moles of Fe3*(aq) in a 100.0 mL sample of an unknown solution, excess KSCN(s) is added to convert all the Fe3*(aq) species into the dark red species FeSCN²*(aq), as represented by the equation above. The absorbance of FESCN2*(aq) at different concentrations is shown in the graph below. 0.50 0.40 0.30 0.20 0.10 5x 10-5 10 x 10-5 Concentration of FeSCN²* (M) If the absorbance of the mixture is 0.20 at 453 nm, how many moles of Fe3+(aq) were present in the 100.0 mL sample? (Assume that any volume change due to adding KSCN is negligible.) О Зх 106 mol O 4x 104 mol 4x 10-6 mol O 2x 105 mol О 3х 104 mol Absorbance at 453 nm
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.8QAP
Related questions
Question
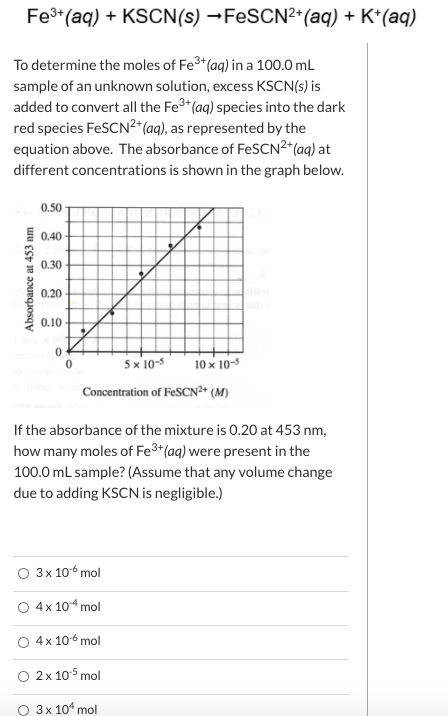
Transcribed Image Text:Fe*(aq) + KSCN(s) →FESCN²+(aq) + K*(aq)
To determine the moles of Fe3*(aq) in a 100.0 mL
sample of an unknown solution, excess KSCN(s) is
added to convert all the Fe3*(aq) species into the dark
red species FeSCN²*(aq), as represented by the
equation above. The absorbance of FESCN2*(aq) at
different concentrations is shown in the graph below.
0.50
0.40
0.30
0.20
0.10
5x 10-5
10 x 10-5
Concentration of FeSCN²* (M)
If the absorbance of the mixture is 0.20 at 453 nm,
how many moles of Fe3+(aq) were present in the
100.0 mL sample? (Assume that any volume change
due to adding KSCN is negligible.)
О Зх 106 mol
O 4x 104 mol
4x 10-6 mol
O 2x 105 mol
О 3х 104 mol
Absorbance at 453 nm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

