Fill in the blank. One mole of any atomic, ionic or molecular substance is it's respective atomic, ionic or molecular mass in grams. Choose the correct option. One mole of water weighs 18 g. The number of moles in 50 g of water is 2.78 The number of molecules in 50 g of water is 6.592x10^23
Fill in the blank. One mole of any atomic, ionic or molecular substance is it's respective atomic, ionic or molecular mass in grams. Choose the correct option. One mole of water weighs 18 g. The number of moles in 50 g of water is 2.78 The number of molecules in 50 g of water is 6.592x10^23
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 2CR: erhaps the most important concept in introductory chemistry concerns what a mole of a substance...
Related questions
Question
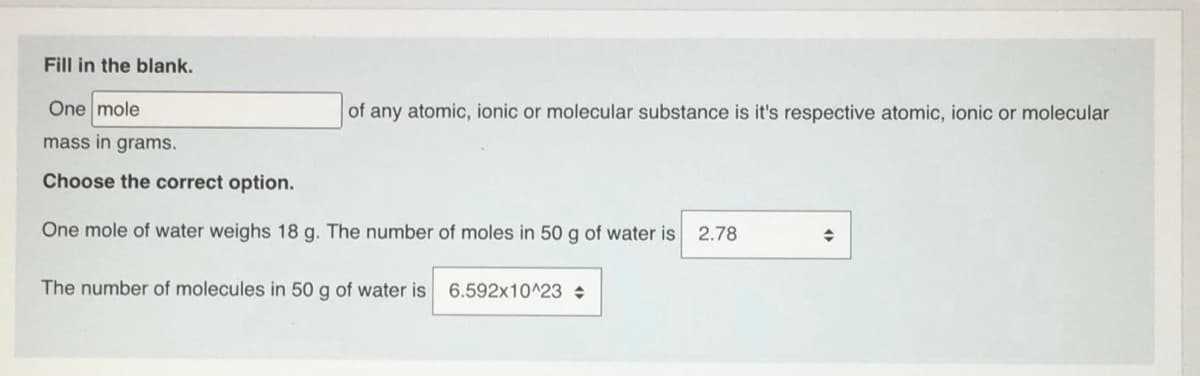
Transcribed Image Text:Fill in the blank.
One mole
of any atomic, ionic or molecular substance is it's respective atomic, ionic or molecular
mass in grams.
Choose the correct option.
One mole of water weighs 18 g. The number of moles in 50 g of water is
2.78
The number of molecules in 50 g of water is
6.592x10^23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning