Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 × 10-¹⁹ J. (a) a photon having a frequency of 6.50 x 10¹7 Hz (b) a photon having a wavelength of 2.50 x 10² nm Step 1 (a) The energy of a photon is given by E = hf, where h = 6.63 x 10-34 Js is Planck's constant and f the frequency of the electromagnetic wave associated with the photon. A photon having frequency f = 6.50 x 10¹7 Hz has an energy, expressed in units of electron volts, of E = hf = ·s) ([ x 10³ ev. 6.63 x 10-34 J. x 1017 Hz 1 eV x 10-19.
Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 × 10-¹⁹ J. (a) a photon having a frequency of 6.50 x 10¹7 Hz (b) a photon having a wavelength of 2.50 x 10² nm Step 1 (a) The energy of a photon is given by E = hf, where h = 6.63 x 10-34 Js is Planck's constant and f the frequency of the electromagnetic wave associated with the photon. A photon having frequency f = 6.50 x 10¹7 Hz has an energy, expressed in units of electron volts, of E = hf = ·s) ([ x 10³ ev. 6.63 x 10-34 J. x 1017 Hz 1 eV x 10-19.
Classical Dynamics of Particles and Systems
5th Edition
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Stephen T. Thornton, Jerry B. Marion
Chapter14: Special Theory Of Relativity
Section: Chapter Questions
Problem 14.42P
Related questions
Question
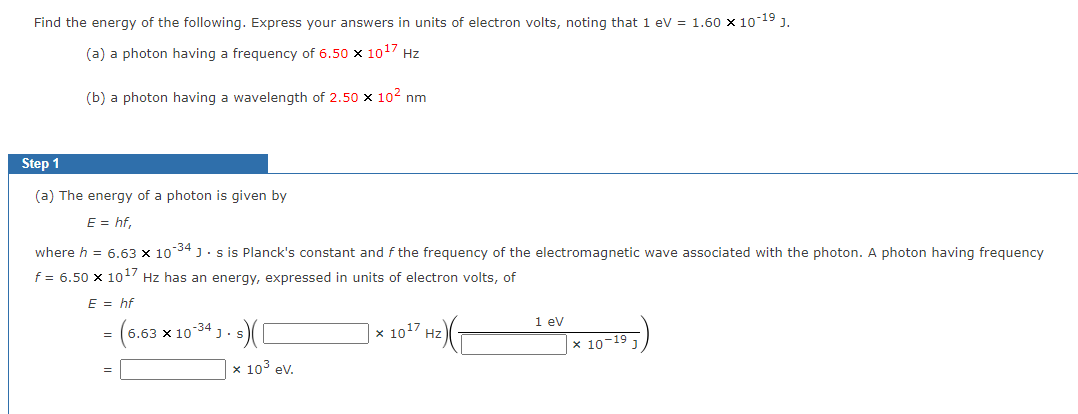
Transcribed Image Text:Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 x 10-19 J.
(a) a photon having a frequency of 6.50 x 10¹7 Hz
(b) a photon having a wavelength of 2.50 x 10² nm
Step 1
(a) The energy of a photon is given by
E = hf,
where h = 6.63 x 10-34 Js is Planck's constant and f the frequency of the electromagnetic wave associated with the photon. A photon having frequency
f = 6.50 x 10¹7 Hz has an energy, expressed in units of electron volts, of
E = hf
= (6.63 × 10-34 J. s) (1
x 10³ ev.
x 10¹7 Hz)
1 eV
x 10-19 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:
9781337399944
Author:
Michael A. Seeds
Publisher:
Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:
9780534408961
Author:
Stephen T. Thornton, Jerry B. Marion
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:
9781337399944
Author:
Michael A. Seeds
Publisher:
Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:
9781337399920
Author:
Michael A. Seeds, Dana Backman
Publisher:
Cengage Learning
