Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 × 10-19 J. (a) a photon having a frequency of 7.80 x 10¹7 Hz eV (b) a photon having a wavelength of 2.00 x 10² nm eV
Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 × 10-19 J. (a) a photon having a frequency of 7.80 x 10¹7 Hz eV (b) a photon having a wavelength of 2.00 x 10² nm eV
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter3: The Quantum Theroy Of Light
Section: Chapter Questions
Problem 40P
Related questions
Question

Transcribed Image Text:Your landscape maintenance person is only allowed by state law to trim branches from trees up to a height of 15 feet without having a special license for tree work. You want to
determine the height of a tree in your back yard to see if he can trim it all the way to the top. On a sunny day, you notice that the tree casts a shadow on the flat ground. Armed
with a tape measure and a protractor, you run out into your back yard. The length of the shadow cast by the tree is 10.6 ft. You quickly set up your protractor and measure that the
Sun is 57.2° above the horizon. What is the height of the tree (in ft)?
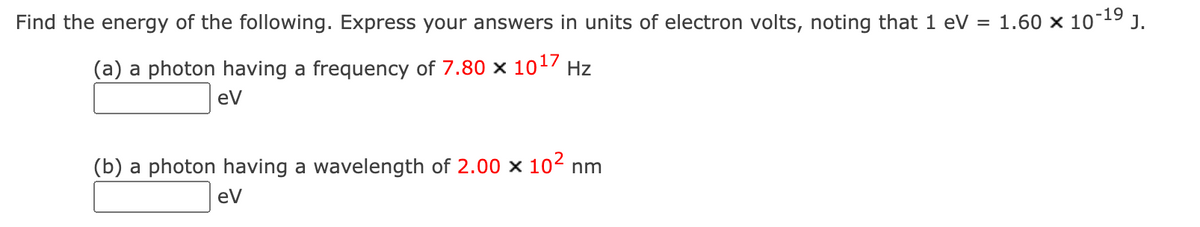
Transcribed Image Text:Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 × 10-¹⁹ J.
(a) a photon having a frequency of 7.80 x 1017 Hz
eV
(b) a photon having a wavelength of 2.00 x 10² nm
eV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax