Chapter10: Energy
Section: Chapter Questions
Problem 65A
Related questions
Question
find the initial temperature of water, (e), (k), (l)
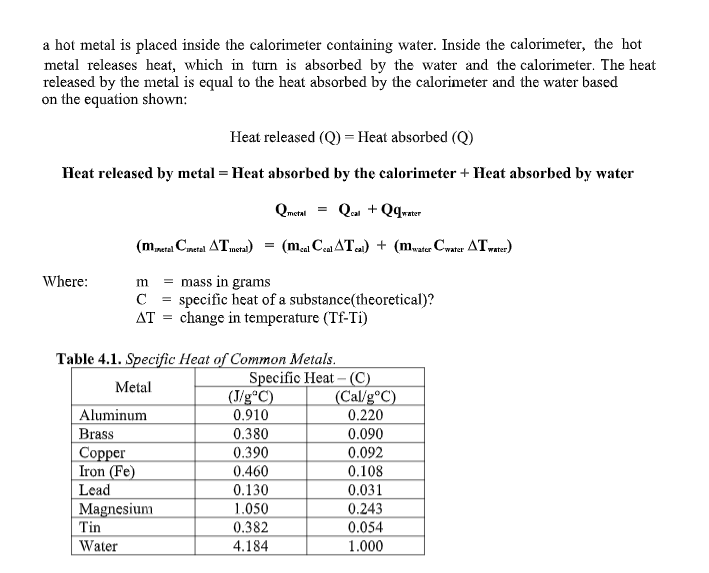
Transcribed Image Text:a hot metal is placed inside the calorimeter containing water. Inside the calorimeter, the hot
metal releases heat, which in turn is absorbed by the water and the calorimeter. The heat
released by the metal is equal to the heat absorbed by the calorimeter and the water based
on the equation shown:
Heat released (Q) = Heat absorbed (Q)
Heat released by metal = Heat absorbed by the calorinmeter + Heat absorbed by water
Qmetal
Qa + Qqrater
(mretal Cmatal ATmeta)
= (m.a Ca ATa) + (mate Cwatur ATrater)
= mass in grams
C = specific heat of a substance(theoretical)?
AT = change in temperature (Tf-Ti)
Where:
m
Table 4.1. Specific Heat of Common Metals.
Specific Heat – (C)
(Cal/g°C)
0.220
Metal
(J/g®C)
0.910
Aluminum
Brass
Copper
Iron (Fe)
Lead
Magnesium
Tin
0.380
0.390
0.090
0.092
0.460
0.108
0.130
0.031
1.050
0.382
0.243
0.054
1.000
Water
4.184
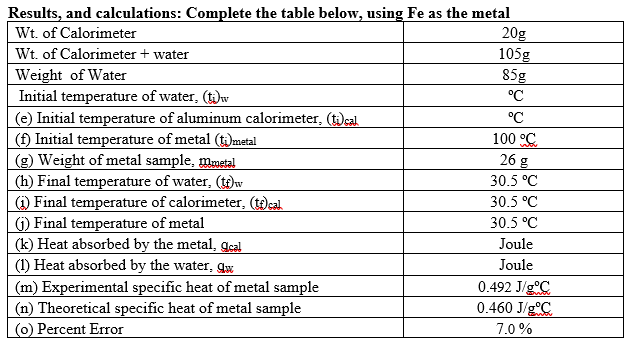
Transcribed Image Text:Results, and calculations: Complete the table below, using Fe as the metal
20g
105g
85g
Wt. of Calorimeter
Wt. of Calorimeter + water
Weight of Water
Initial temperature of water, (t)v
°C
(e) Initial temperature of aluminum calorimeter, (t)eal
(f) Initial temperature of metal (t)metal
(g) Weight of metal sample, mmetal
(h) Final temperature of water, (t)w
(i) Final temperature of calorimeter, (t)cal
G) Final temperature of metal
(k) Heat absorbed by the metal, gcal
(1) Heat absorbed by the water, gw
°C
100 °C
26 g
30.5 °C
30.5 °C
30.5 °C
Joule
Joule
(m) Experimental specific heat of metal sample
0.492 J/gC
(n) Theoretical specific heat of metal sample
0.460 J/gC
(0) Percent Error
7.0 %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning