: Find the percentage of each problem. Balance it if necessarry. Show your solution. (kindly refer to the picture on how must the solution look like) 2. 2Fe + 2CuSO8 -----> 2Cu + 2FeSO8
: Find the percentage of each problem. Balance it if necessarry. Show your solution. (kindly refer to the picture on how must the solution look like) 2. 2Fe + 2CuSO8 -----> 2Cu + 2FeSO8
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 86E: During the discussion of gaseous diffusion for enriching uranium, it was claimed that 235UF6...
Related questions
Question
Hi! Can you help me with these?
Assignment: Find the percentage of each problem. Balance it if necessarry. Show your solution. (kindly refer to the picture on how must the solution look like)
2. 2Fe + 2CuSO8 -----> 2Cu + 2FeSO8
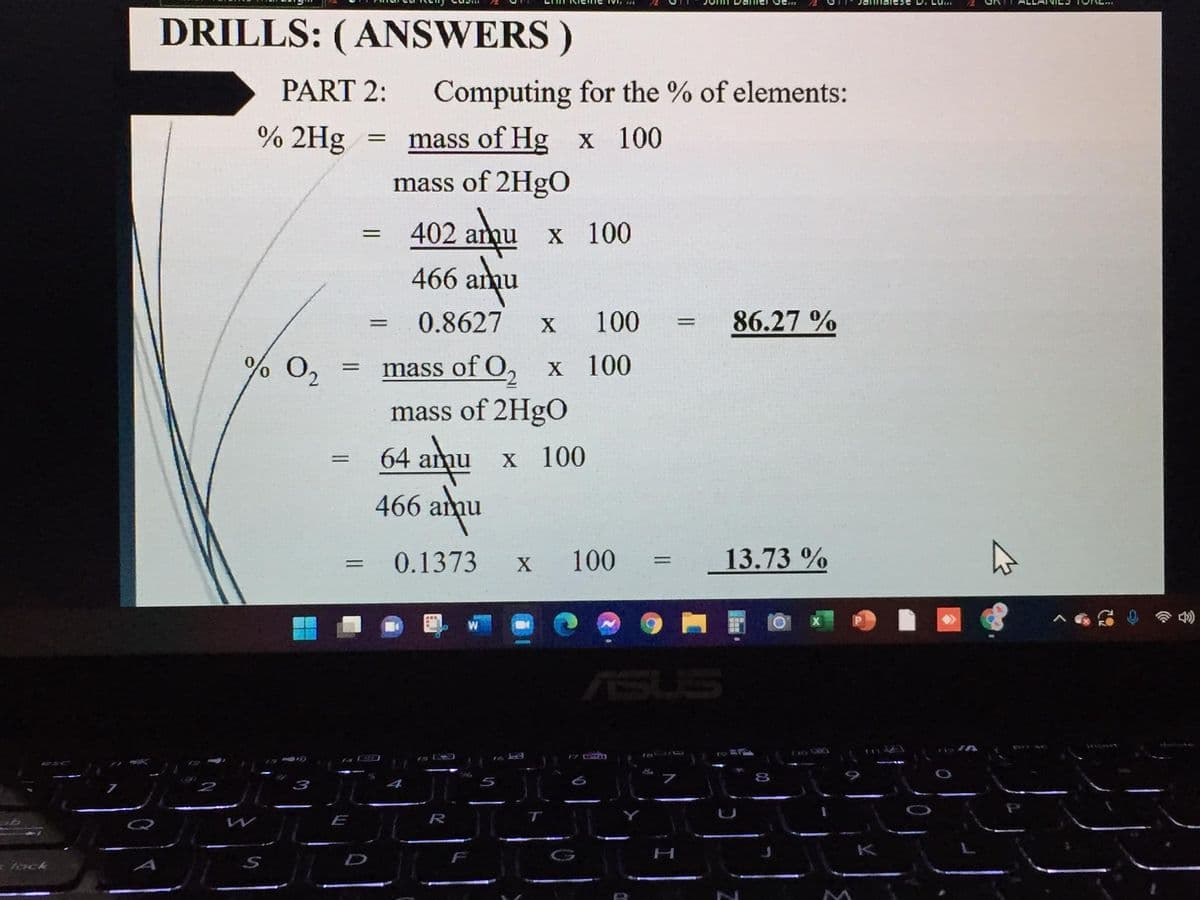
Transcribed Image Text:DRILLS: (ANSWERS )
PART 2:
Computing for the % of elements:
% 2Hg
mass of Hg x 100
mass of 2HgO
402 anqu
466 anu
х 100
0.8627
100
86.27 %
% 0,
mass of O, x 100
mass of 2HgO
64 amu
466 amu
X 100
0.1373
100
13.73 %
%3|
X
ASUS
4S LDD
E
R
- lock

Transcribed Image Text:Remaining Meeting Time: 02:39
Equation:
Na
Cl
NaCl
Formula:
NAt. wt
Nno. of atoms
Na =
Naat, wt X Nano. of atoms
23 amu x 1:
23 ати
CI = Cl,
Clat, wt X CIno. of atoms
35 amu x I
= 35 amu
At. wt
Na
+
Cl
NaCl
(23 amu)
(35 amu)
(58 amu)
02
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax