Which of the given statements are CORRECT for an indicator that ionizes as shown below (Ka=1.0 x 10-4)? (1) The predominant color in its acid range is yellow. (2) In the middle of the pH range of its color change a solution containing the indicator will probably be orange. (3) At pH = 7.00, a solution containing this indicator (and no other colored species) will be red. (4) At pH = 7.00, most of the indicator is in the un-ionized form. (5) The pH at which
Which of the given statements are CORRECT for an indicator that ionizes as shown below (Ka=1.0 x 10-4)? (1) The predominant color in its acid range is yellow. (2) In the middle of the pH range of its color change a solution containing the indicator will probably be orange. (3) At pH = 7.00, a solution containing this indicator (and no other colored species) will be red. (4) At pH = 7.00, most of the indicator is in the un-ionized form. (5) The pH at which
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter24: Biochemistry
Section: Chapter Questions
Problem 25PS
Related questions
Question
Which of the given statements are CORRECT for an indicator that ionizes as shown below (Ka=1.0 x 10-4)?
(1) The predominant color in its acid range is yellow.
(2) In the middle of the pH range of its color change a solution containing the indicator will probably be orange.
(3) At pH = 7.00, a solution containing this indicator (and no other colored species) will be red.
(4) At pH = 7.00, most of the indicator is in the un-ionized form.
(5) The pH at which the indicator changes color is pH = 4.
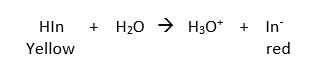
Transcribed Image Text:HIn
H20 → H30*
In
+
Yellow
red
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning