First, draw out the structures in the following reaction to identify the bonds being broken and the ones being formed. Upload the drawing here. CH4 (g) + 20₂ (g) --> CO₂ (g) + 2H₂O (g) Use the editor to format your answer
First, draw out the structures in the following reaction to identify the bonds being broken and the ones being formed. Upload the drawing here. CH4 (g) + 20₂ (g) --> CO₂ (g) + 2H₂O (g) Use the editor to format your answer
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 25CTQ: How many H’s are lost from the molecular formula of pentane if a double bond is introduced(changing...
Related questions
Question
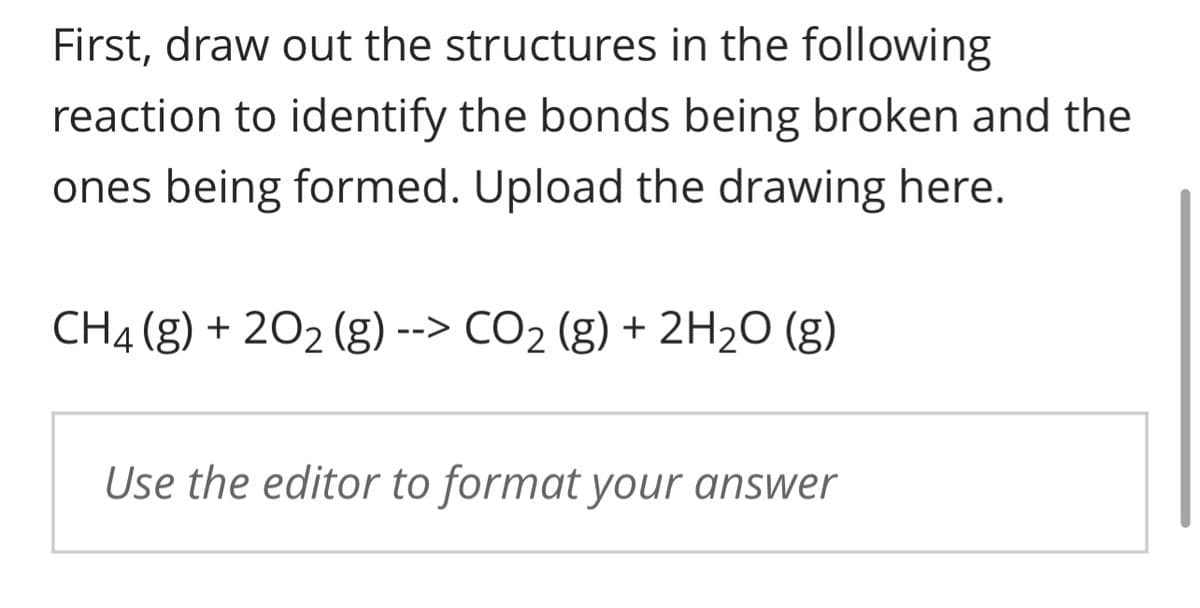
Transcribed Image Text:First, draw out the structures in the following
reaction to identify the bonds being broken and the
ones being formed. Upload the drawing here.
CH4 (g) + 20₂ (g) --> CO₂ (g) + 2H₂O (g)
Use the editor to format your answer
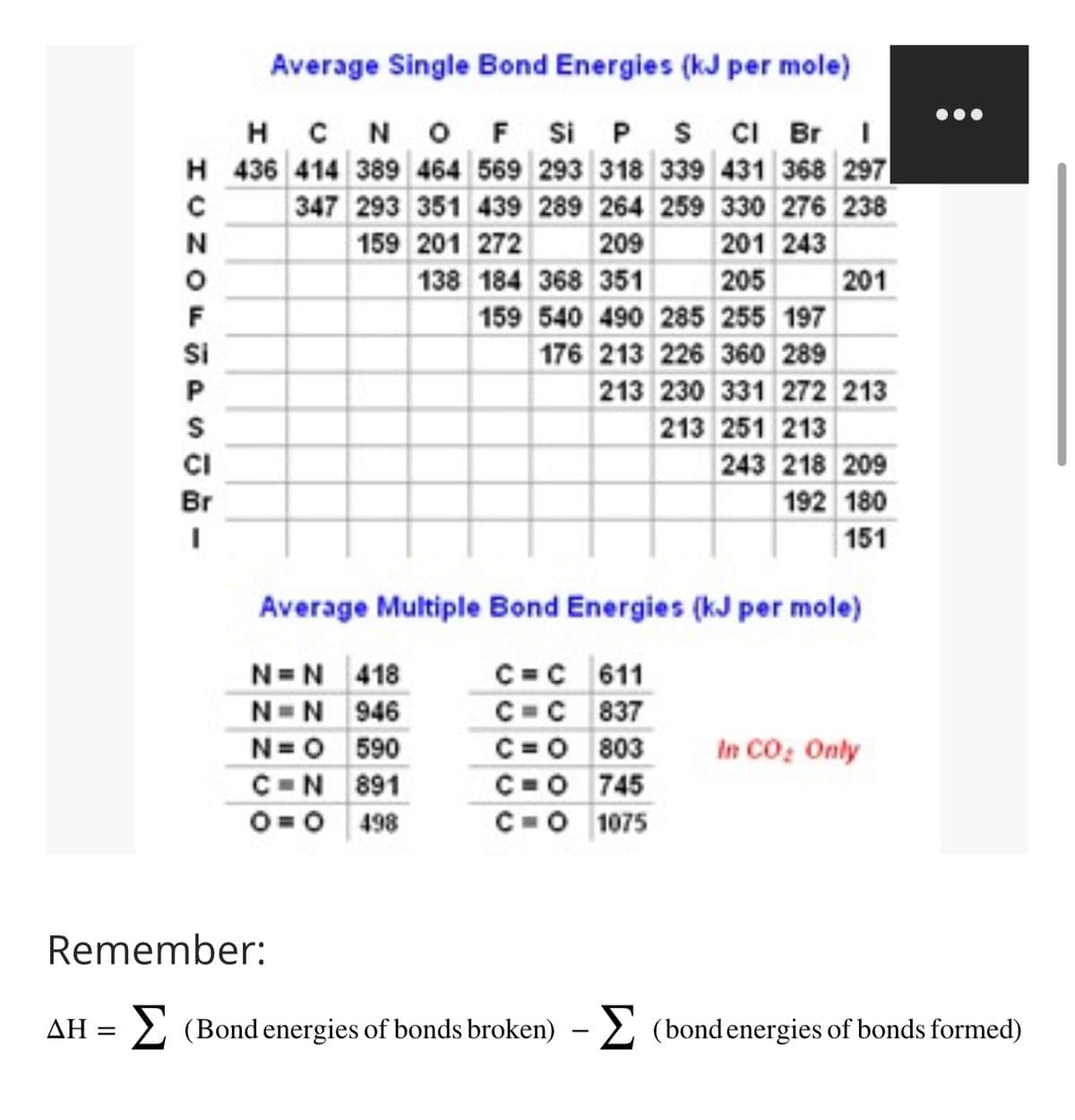
Transcribed Image Text:Average Single Bond Energies (kJ per mole)
HCN OF Si P S Cl Br I
H 436 414 389 464 569 293 318 339 431 368 297
347 293 351 439 289 264 259 330 276 238
159 201 272
209
201 243
138 184 368 351
205
159 540 490 285 255 197
176 213 226 360 289
HCZOFSPST-
Si
CI
Br
201
213 230 331 272 213
213 251 213
243 218 209
192 180
151
Average Multiple Bond Energies (kJ per mole)
N=N418
C=C 611
N=N 946
C=C 837
N=0 590
C=0|803
C-N 891
C=0 745
0 0 498
C=O 1075
In CO₂ Only
Remember:
ΔΗ = Σ (Bondenergies of bonds broken) - Σ (bondenergies of bonds formed)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning