Fo 4. Carry out the following conversions. Use the textbook to obtain conversion values between English and metric. Please have the metric prefixes memorized. Problem Actual answer You purchased 4 quarts of milk at the store. How many milliliters (mL) of milk is that? What mass in kg does a 50 lb bag of dog food have? ii 01X 9179 Yvnn ii How many inches are in 5.95 X 102 km? How many nm are in 5.2 X 102 km? iv 1. Tae How many ms are in 1.25 hours? V vi How many mm are in 1.28 X 105 mi? How much will a 5.2 mg nickel weigh in vii lbs?
Fo 4. Carry out the following conversions. Use the textbook to obtain conversion values between English and metric. Please have the metric prefixes memorized. Problem Actual answer You purchased 4 quarts of milk at the store. How many milliliters (mL) of milk is that? What mass in kg does a 50 lb bag of dog food have? ii 01X 9179 Yvnn ii How many inches are in 5.95 X 102 km? How many nm are in 5.2 X 102 km? iv 1. Tae How many ms are in 1.25 hours? V vi How many mm are in 1.28 X 105 mi? How much will a 5.2 mg nickel weigh in vii lbs?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 39QAP: A lap in most tracks in the United States is 0.25 mi (English lap). In most countries that use the...
Related questions
Question
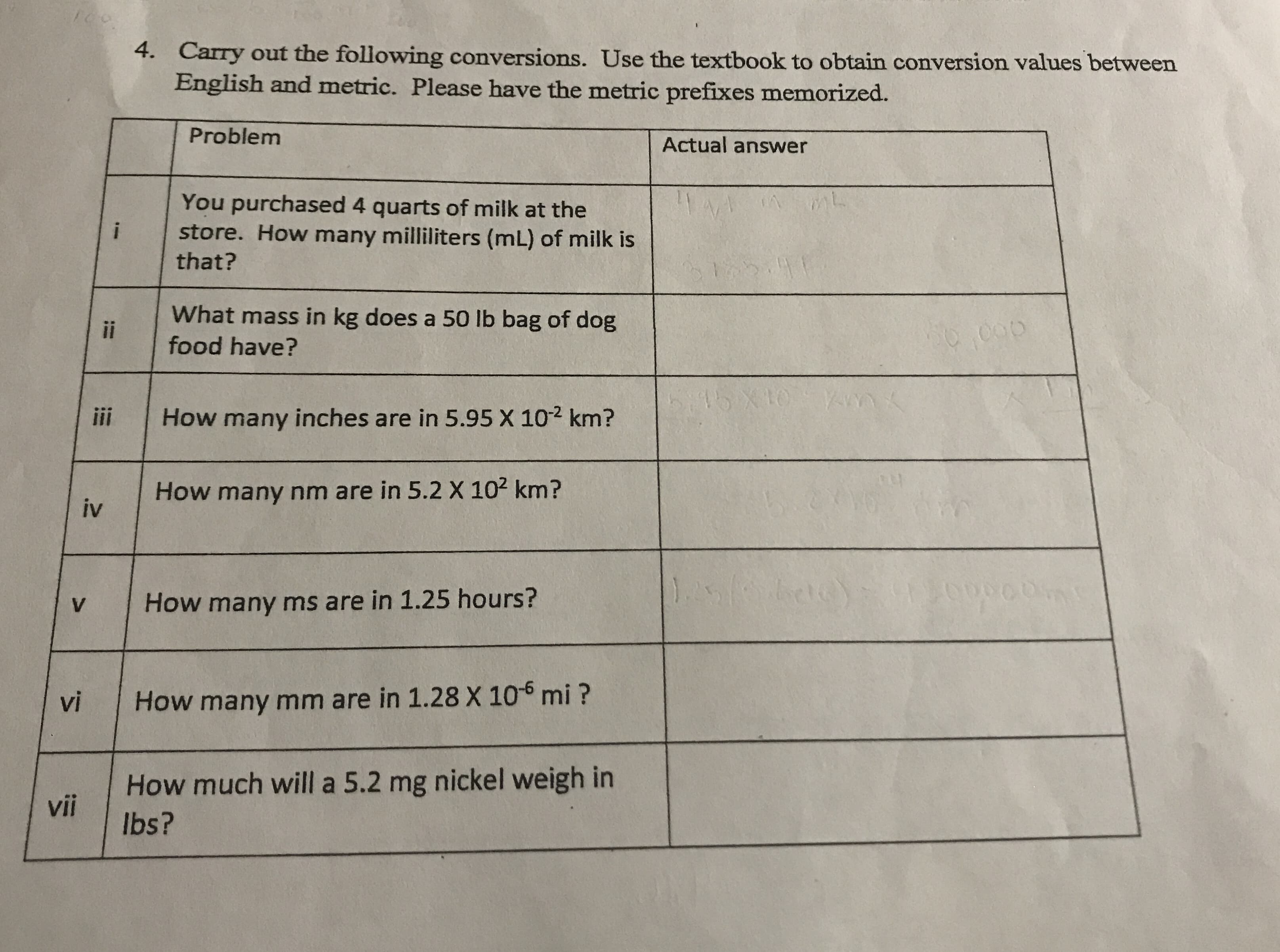
Transcribed Image Text:Fo
4. Carry out the following conversions. Use the textbook to obtain conversion values between
English and metric. Please have the metric prefixes memorized.
Problem
Actual answer
You purchased 4 quarts of milk at the
store. How many milliliters (mL) of milk is
that?
What mass in kg does a 50 lb bag of dog
food have?
ii
01X 9179
Yvnn
ii
How many inches are in 5.95 X 102 km?
How many nm are in 5.2 X 102 km?
iv
1. Tae
How many ms are in 1.25 hours?
V
vi
How many mm are in 1.28 X 105 mi?
How much will a 5.2 mg nickel weigh in
vii
lbs?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
