Following is an example of a type of reaction known as a Diels-Alder reaction 1,3-Pentadiene Ethylene 3-Methylcyclohexene (a racemic mixture) The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is one of the few ways that chemists have to form two new carbon-carbon bonds in a single reaction. Given what you know about the relative strengths of carbon-carbon sigma and pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic? Explain your reasoning.
Following is an example of a type of reaction known as a Diels-Alder reaction 1,3-Pentadiene Ethylene 3-Methylcyclohexene (a racemic mixture) The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is one of the few ways that chemists have to form two new carbon-carbon bonds in a single reaction. Given what you know about the relative strengths of carbon-carbon sigma and pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic? Explain your reasoning.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter6: Reactions Of Alkenes
Section: Chapter Questions
Problem 6.53P
Related questions
Question
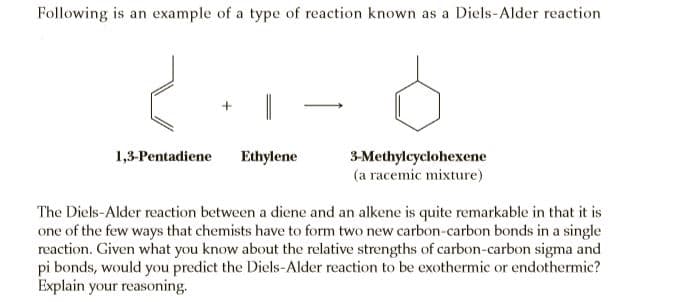
Transcribed Image Text:Following is an example of a type of reaction known as a Diels-Alder reaction
1,3-Pentadiene
Ethylene
3-Methylcyclohexene
(a racemic mixture)
The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is
one of the few ways that chemists have to form two new carbon-carbon bonds in a single
reaction. Given what you know about the relative strengths of carbon-carbon sigma and
pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic?
Explain your reasoning.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
