For a given acid HA, it was determined that at pH 6.0 the concentration of the conjugate base [A] was 0.075 M and the acid [HA] was 0.025 M. What percent of this acid is ionized at pH 6.0? What is the pKa of this acid? What pH would this acid be 50% ionized?
For a given acid HA, it was determined that at pH 6.0 the concentration of the conjugate base [A] was 0.075 M and the acid [HA] was 0.025 M. What percent of this acid is ionized at pH 6.0? What is the pKa of this acid? What pH would this acid be 50% ionized?
Chapter20: Carboxylic Acids And Nitriles
Section20.4: Substituent Effects On Acidity
Problem 7P: Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a...
Related questions
Question
![For a given acid HA, it was determined that at pH 6.0 the concentration of the conjugate base [A]
was 0.075 M and the acid [HA] was 0.025 M.
What percent of this acid is ionized at pH 6.0?
What is the pKa of this acid?
What pH would this acid be 50% lonized?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0176354b-6380-4b3b-a690-4fa0d9bd1e4d%2F48b1fdf2-2204-4d8f-80b1-967c9b413081%2Fs1szmy_processed.jpeg&w=3840&q=75)
Transcribed Image Text:For a given acid HA, it was determined that at pH 6.0 the concentration of the conjugate base [A]
was 0.075 M and the acid [HA] was 0.025 M.
What percent of this acid is ionized at pH 6.0?
What is the pKa of this acid?
What pH would this acid be 50% lonized?
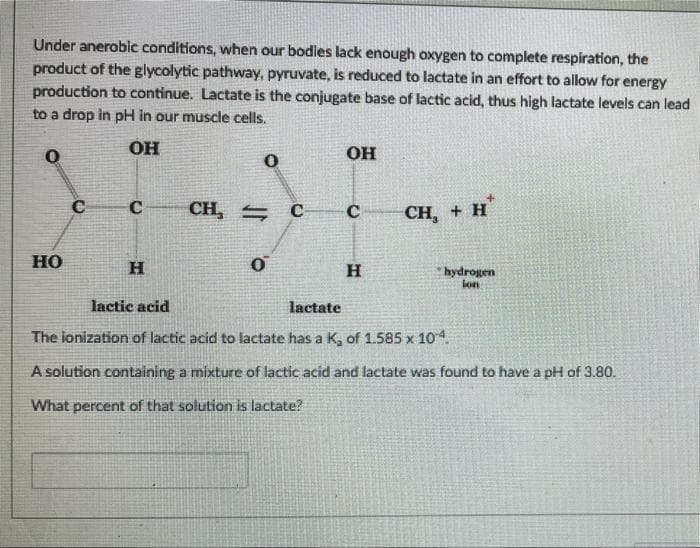
Transcribed Image Text:Under anerobic conditions, when our bodies lack enough oxygen to complete respiration, the
product of the glycolytic pathway, pyruvate, is reduced to lactate in an effort to allow for energy
production to continue. Lactate is the conjugate base of lactic acid, thus high lactate levels can lead
to a drop in pH in our muscle cells.
0
OH
OH
0
C
сH, - с
C
CH, + H
HO
H
0
H
hydrogen
ion
lactic acid
lactate
The ionization of lactic acid to lactate has a K₂ of 1.585 x 10-4.
A solution containing a mixture of lactic acid and lactate was found to have a pH of 3.80.
What percent of that solution is lactate?
с
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



