For a given Cp as a function of T: 41.87 t + 100 Cp = 2.093 + Where Cp is in J deg C and t is in deg C The system's volume increases from 2,000 to 2,400 cubic centimeter due to it being heated a 1 atmosphere while the temperature raises from standard to 100 deg C. Determine: A.) the heat transferred (J) B.) the change in internal energy of the system (J)
For a given Cp as a function of T: 41.87 t + 100 Cp = 2.093 + Where Cp is in J deg C and t is in deg C The system's volume increases from 2,000 to 2,400 cubic centimeter due to it being heated a 1 atmosphere while the temperature raises from standard to 100 deg C. Determine: A.) the heat transferred (J) B.) the change in internal energy of the system (J)
Automotive Technology: A Systems Approach (MindTap Course List)
6th Edition
ISBN:9781133612315
Author:Jack Erjavec, Rob Thompson
Publisher:Jack Erjavec, Rob Thompson
Chapter3: Basic Theories And Math
Section: Chapter Questions
Problem 2RQ: In what four states does matter exist? Cite examples of each.
Related questions
Question
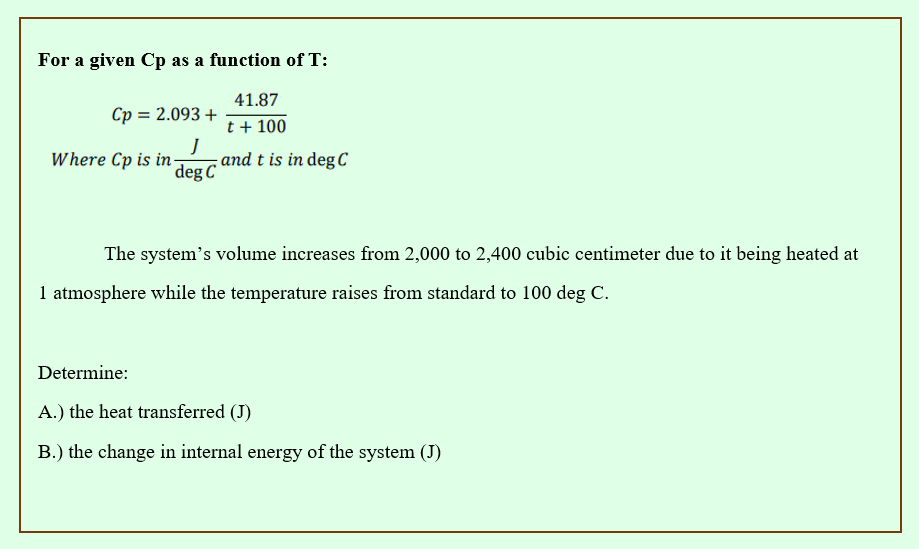
Transcribed Image Text:For a given Cp as a function of T:
41.87
t + 100
Cp = 2.093 +
Where Cp is in
J
deg C
and t is in deg C
The system's volume increases from 2,000 to 2,400 cubic centimeter due to it being heated at
1 atmosphere while the temperature raises from standard to 100 deg C.
Determine:
A.) the heat transferred (J)
B.) the change in internal energy of the system (J)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning

Automotive Technology: A Systems Approach (MindTa…
Mechanical Engineering
ISBN:
9781133612315
Author:
Jack Erjavec, Rob Thompson
Publisher:
Cengage Learning