For a hydrogen atom, calculate the wavelength of light (in m) that would be emitted for the orbital transition of n(initial) = 3 to n(final) = 1. Submit an answer to four significant figures. The Rydberg constant is 1.09678 x 107 m1. m 1 4 C 8 +/- x 100 3. 5
For a hydrogen atom, calculate the wavelength of light (in m) that would be emitted for the orbital transition of n(initial) = 3 to n(final) = 1. Submit an answer to four significant figures. The Rydberg constant is 1.09678 x 107 m1. m 1 4 C 8 +/- x 100 3. 5
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter6: The Periodic Table And Atomic Structure
Section: Chapter Questions
Problem 6.8PAE: 6.8 Calculate the wavelength in meters, of radiation of the following frequencies. (a) 5.001015s1...
Related questions
Question
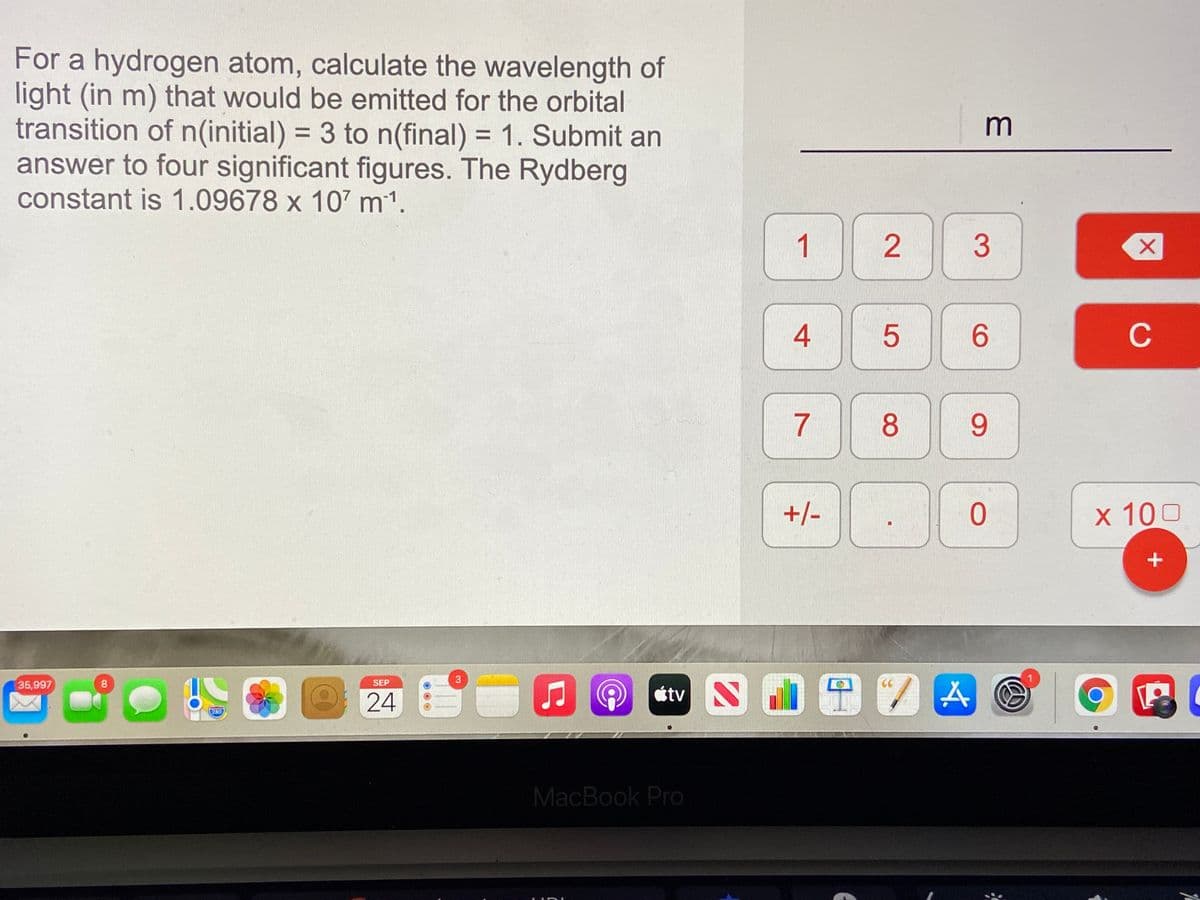
Transcribed Image Text:For a hydrogen atom, calculate the wavelength of
light (in m) that would be emitted for the orbital
transition of n(initial) = 3 to n(final) = 1. Submit an
answer to four significant figures. The Rydberg
constant is 1.09678 x 107 m1.
m
%3D
1
3
4
6.
C
8
9.
+/-
x 100
étv S
20田7囚
35,997
8
SEP
24
280
MacBook Pro
2.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
