For answers to calculations, use the correct number of significant figures. The percentage of sodium hydrogen carbonate (Nal ICO, 84.01 gimol) in a powder for stomach upsets is found by titrating with 0.275 M hydrochloric acid (HCI). The balanced equation lor the reaction that takes place is NaHCOs(s) + H"(aq) - Na"(aq) + CO:(9) + H;O) a) If 16.5 mL of hydrochloric acid is required to react with 0.500 g of the sample, what is he percentage by mass of sodium hydrogen carbonate in the sample? ANSWER: % NaHCOs by mass b) The reaction between hydrochloric acid and sodium hydrogen carbonate is neutralization c) Aside from treating stomach upsets, sodium hydrogen carbonate is also added to make bread dough rise, and commonly placed in refrigerators to remove unwanted odors Its common name is Type to searc from your seved nfo
For answers to calculations, use the correct number of significant figures. The percentage of sodium hydrogen carbonate (Nal ICO, 84.01 gimol) in a powder for stomach upsets is found by titrating with 0.275 M hydrochloric acid (HCI). The balanced equation lor the reaction that takes place is NaHCOs(s) + H"(aq) - Na"(aq) + CO:(9) + H;O) a) If 16.5 mL of hydrochloric acid is required to react with 0.500 g of the sample, what is he percentage by mass of sodium hydrogen carbonate in the sample? ANSWER: % NaHCOs by mass b) The reaction between hydrochloric acid and sodium hydrogen carbonate is neutralization c) Aside from treating stomach upsets, sodium hydrogen carbonate is also added to make bread dough rise, and commonly placed in refrigerators to remove unwanted odors Its common name is Type to searc from your seved nfo
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 20QAP: Many over-the-counter antacid tablets are now formulated using calcium carbonate as the active...
Related questions
Question
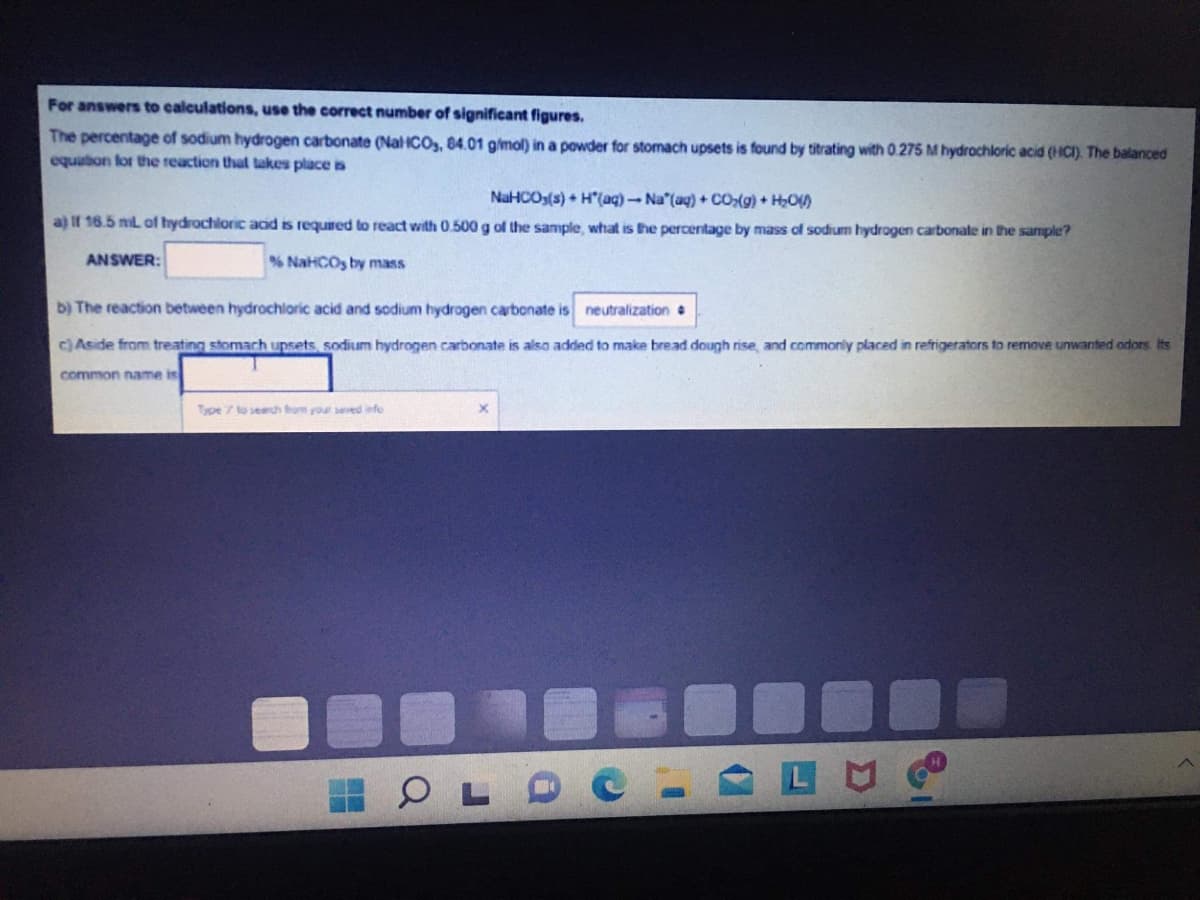
Transcribed Image Text:For answers to calculations, use the correct number of significant figures.
The percentage of sodium hydrogen carbonate (NalHCOs, 84.01 gimol) in a powder for stomach upsets is found by titrating with 0.275 M hydrochloric acid (HCI). The balanced
equution for the reaction that takes place is
NaHCOs(s) + H"(aq)-Na (ag) + cO(g) + H,O)
a) If 16.5 mil of hydrochloric acd is required to react with 0.500 g of the sample, what is the percentage by mass of sodium hydrogen carbonate in the sample?
ANSWER:
% NaHCOs by mass
b) The reaction between hydrochlaric acid and sodium hydrogen carbonate is neutralization
c) Aside from treating stomach upsets, sodium hydrogen carbonate is also added to make bread dough rise, and commonly placed in refrigerators to remove unwanted odors Its
common name is
Type 7 to search fom your seved iefo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
