Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.51P
Related questions
Question
Which group of side chains is most likely to take part in the catalysis during an enzymatic reaction? Substantiate the answer.
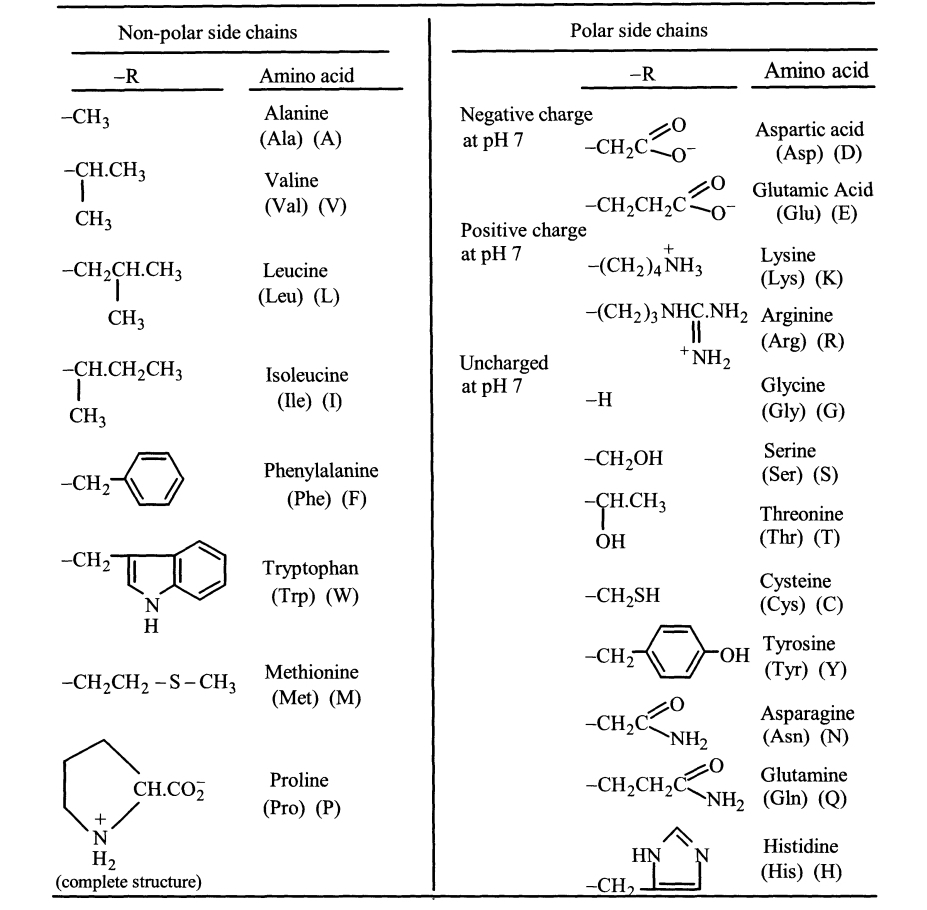
Transcribed Image Text:Non-polar side chains
Polar side chains
-R
Amino acid
-R
Amino acid
Alanine
Negative charge
at pH 7
-CH3
Aspartic acid
(Asp) (D)
(Ala) (A)
-CH2C.
-CH.CH3
Valine
Glutamic Acid
(Val) (V)
-CH2CH2C:
(Glu) (E)
CH3
Positive charge
at pH 7
-CH2CH.CH3
-(CH2)4 NH3
Lysine
(Lys) (K)
Leucine
(Leu) (L)
CH3
-(CH2)3 NHC.NH, Arginine
(Arg) (R)
* NH2
--CH.CH2CH3
Uncharged
at pH 7
Isoleucine
Glycine
(Gly) (G)
(Ile) (I)
-H
CH3
Serine
-CH2OH
Phenylalanine
(Phe) (F)
-CH2
(Ser) (S)
-CH.CH3
Threonine
OH
(Thr) (T)
-CH2
Tryptophan
(Trp) (W)
Cysteine
(Сys) (C)
-CH2SH
N
H
Tyrosine
(Тyг) (Ү)
-CH,
FOH
Methionine
-CH2CH2 -S-CH3
(Met) (M)
-CH,C
`NH2
Asparagine
(Asn) (N)
CH.CO2
Proline
-CH2CH,C
Glutamine
(Pro) (P)
NH2 (Gln) (Q)
Histidine
HN
N.
H2
(complete structure)
(His) (H)
-CH,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 7 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning