For Cobalt - 59, determine the mass defect (in g/ mol) and binding energy (in kJ/mal). Table 20.3 Masses of Some Nuclei and Other Atomic Particles Symbol Mass (amu) Symbls Mass (amu 0. 0.000549 Co 27 59 58.9184 e 1.00867 Ni 28 58 57.9199 206 207 205.9295 82 82 82 Ph 1.00728 2.01345 3.01550 H or p 206.9309 207.9316 2. 208 3.01493 4.00150 209.9368 217.9628 84 210 218 He 3. Po 4. 84 6. 7. 6.01347 7.01435 221.9703 225.9771 Rn 222 226 Li 86 Ra 88 Be 9. 9.00999 Th 90 230 229.9837 10.0102 11.0066 10 90 91 234 234 233.9942 233.9931 11 Pa 6. 12 13 11.9967 13.0001 92 232.9890 233.9904 233 6. 92 234 16 15.9905 92 235 234.9934 Cr 52 51.9273 92 238 238.0003 Fe 56 55.9206 Pu 94 239 239.0006 22 334 n50o8 $8
For Cobalt - 59, determine the mass defect (in g/ mol) and binding energy (in kJ/mal). Table 20.3 Masses of Some Nuclei and Other Atomic Particles Symbol Mass (amu) Symbls Mass (amu 0. 0.000549 Co 27 59 58.9184 e 1.00867 Ni 28 58 57.9199 206 207 205.9295 82 82 82 Ph 1.00728 2.01345 3.01550 H or p 206.9309 207.9316 2. 208 3.01493 4.00150 209.9368 217.9628 84 210 218 He 3. Po 4. 84 6. 7. 6.01347 7.01435 221.9703 225.9771 Rn 222 226 Li 86 Ra 88 Be 9. 9.00999 Th 90 230 229.9837 10.0102 11.0066 10 90 91 234 234 233.9942 233.9931 11 Pa 6. 12 13 11.9967 13.0001 92 232.9890 233.9904 233 6. 92 234 16 15.9905 92 235 234.9934 Cr 52 51.9273 92 238 238.0003 Fe 56 55.9206 Pu 94 239 239.0006 22 334 n50o8 $8
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter24: Nuclear Chemistry
Section: Chapter Questions
Problem 55A
Related questions
Question
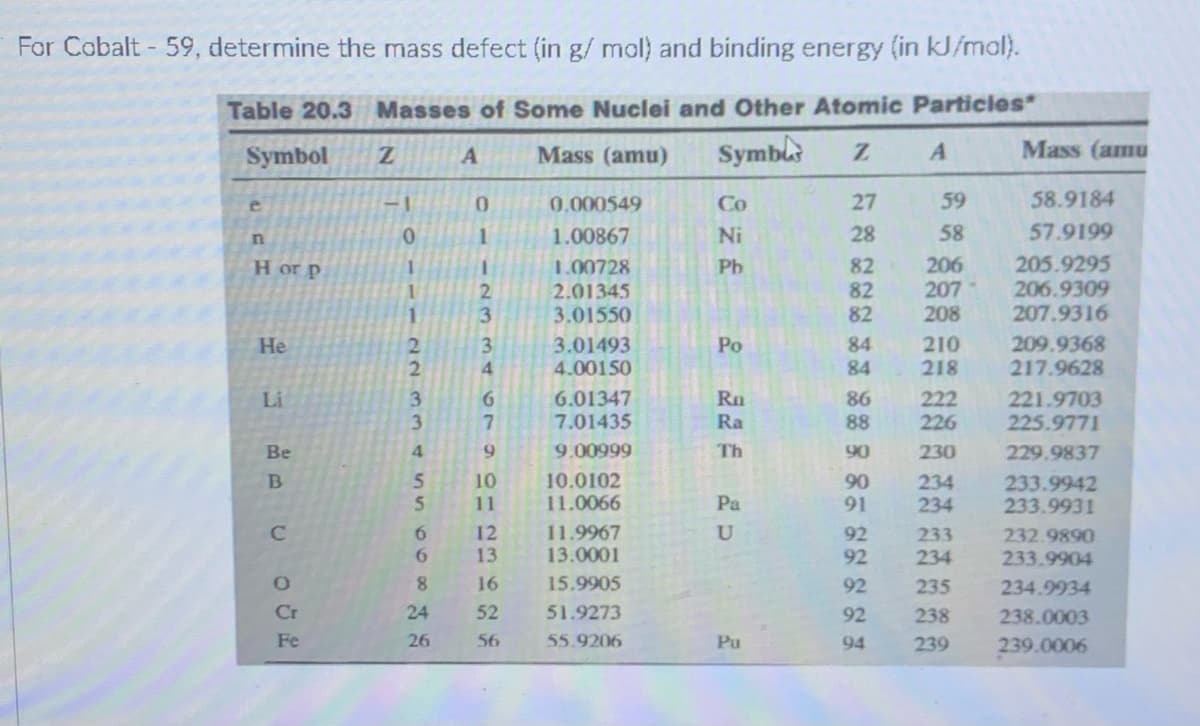
Transcribed Image Text:For Cobalt - 59, determine the mass defect (in g/ mol) and binding energy (in kJ/mal).
Table 20.3 Masses of Some Nuclei and Other Atomic Particles*
Symbol
Mass (amu)
Symbl
Mass (amu
-1
0.
0,000549
Co
27
59
58.9184
1.
1.00867
Ni
28
58
57.9199
1.00728
2.01345
3.01550
82
82
82
206
207
208
205.9295
206.9309
207.9316
H or p
Pb
2.
3.
3.01493
4.00150
210
218
209.9368
217.9628
3
Po
84
84
He
3.
3.
6.01347
7.01435
6.
Rn
86
88
222
226
221.9703
225.9771
Li
Ra
Be
4.
9.
9.00999
Th
90
230
229.9837
10
10.0102
11.0066
90
234
234
233.9942
233.9931
11
Pa
91
12
13
C
11.9967
92
233
232.9890
13.0001
92
234
233.9904
8.
16
15.9905
92
235
234.9934
Cr
24
52
51.9273
92
238
238.0003
Fe
26
56
55.9206
Pu
94
239
239.0006
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
