Selenium-82 undergoes double beta decay: 82 Se 34 → Kr + 2 je + 27 This low-probability process occurs with a half-life of 3.5 x 1027 s, one of the longest half-lives ever measured. Estimate the activity in an 82.0-g (1.00 mol) sample of this isotope. How many 82Se nuclei decay in a day?
Selenium-82 undergoes double beta decay: 82 Se 34 → Kr + 2 je + 27 This low-probability process occurs with a half-life of 3.5 x 1027 s, one of the longest half-lives ever measured. Estimate the activity in an 82.0-g (1.00 mol) sample of this isotope. How many 82Se nuclei decay in a day?
Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.19E
Related questions
Question
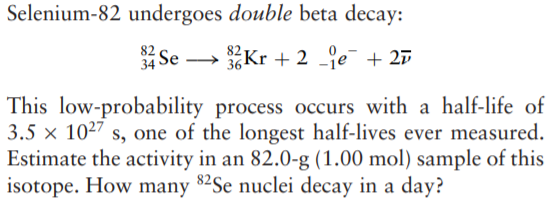
Transcribed Image Text:Selenium-82 undergoes double beta decay:
82 Se
34
→ Kr + 2 je + 27
This low-probability process occurs with a half-life of
3.5 x 1027 s, one of the longest half-lives ever measured.
Estimate the activity in an 82.0-g (1.00 mol) sample of this
isotope. How many 82Se nuclei decay in a day?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
