For covalent molecules, a nitrogen atom prefers to form a total of, -- single bond(s), while an oxygen atom prefers to form a total of _ single bond(s). 3; 4 4; 2 2; 1 O 1; 2 3; 2
For covalent molecules, a nitrogen atom prefers to form a total of, -- single bond(s), while an oxygen atom prefers to form a total of _ single bond(s). 3; 4 4; 2 2; 1 O 1; 2 3; 2
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 3QRT
Related questions
Question
Please provide the reason why the chosen answer is correct!
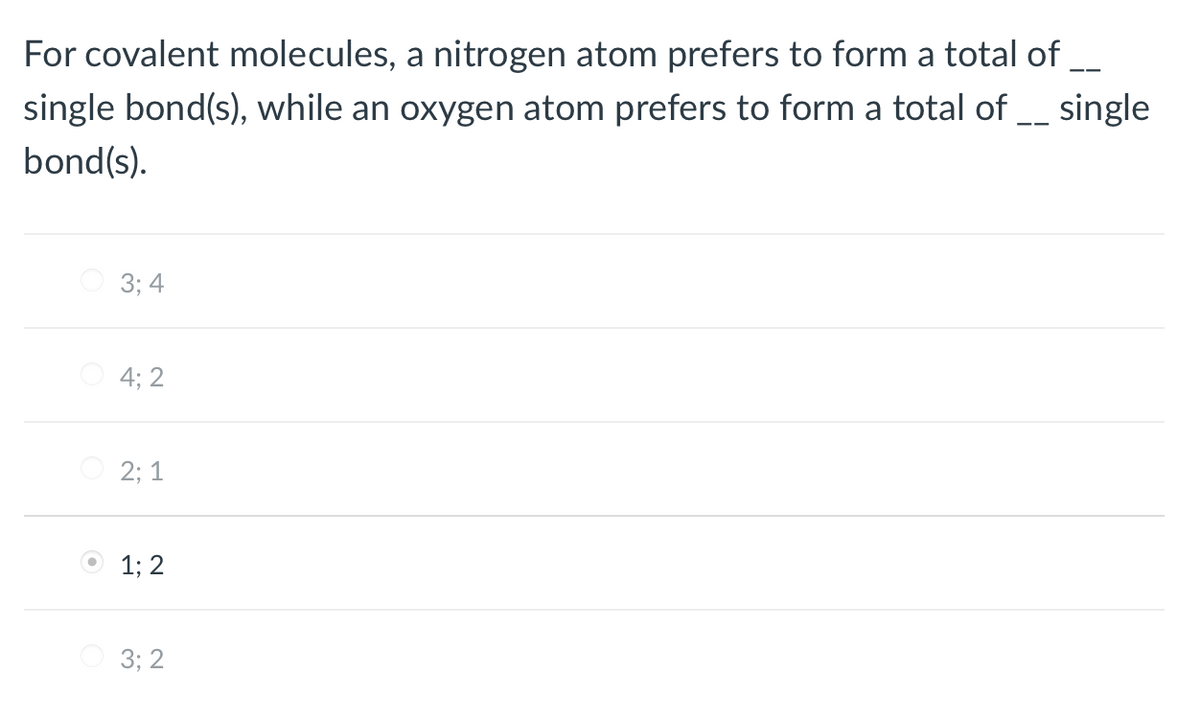
Transcribed Image Text:For covalent molecules, a nitrogen atom prefers to form a total of _
single bond(s), while an oxygen atom prefers to form a total of __ single
bond(s).
3; 4
4; 2
O 2; 1
O 1; 2
3; 2
Expert Solution
Step 1
Covalent molecules are formed by the sharing of electrons between the atoms.
The number of single bonds formed by the atoms can be decided by its valency.
For Nitrogen:
Atomic number =7
Electronic configuration: 1s2 2s2 2p3
Valence electrons =5
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning