For divers going to great depths, the composition of the air in the tank must be modified. The ideal composition is to have approximately the same number of O2 molecules per unit volume as in surface air (to avoid oxygen poisoning), and to use helium instead of nitrogen for the remainder of the gas (to avoid nitrogen narcosis, which results from nitrogen dissolving in the bloodstream). Of the molecules in dry surface air, 78.0% are N2, 21.0% are O2, and 1.00% are Ar. (Assume that the density of seawater is 1025 kg/m³ and the temperature is 20.0°C.) Density of dry air at 20.0°C is 1.20 kg/m3. Molar mass of N2 is 14.007 g/mol, O2 is 15.999 g/mol, and Ar is 39.948 g/mol. (See Table B.7.)
For divers going to great depths, the composition of the air in the tank must be modified. The ideal composition is to have approximately the same number of O2 molecules per unit volume as in surface air (to avoid oxygen poisoning), and to use helium instead of nitrogen for the remainder of the gas (to avoid nitrogen narcosis, which results from nitrogen dissolving in the bloodstream). Of the molecules in dry surface air, 78.0% are N2, 21.0% are O2, and 1.00% are Ar. (Assume that the density of seawater is 1025 kg/m³ and the temperature is 20.0°C.) Density of dry air at 20.0°C is 1.20 kg/m3. Molar mass of N2 is 14.007 g/mol, O2 is 15.999 g/mol, and Ar is 39.948 g/mol. (See Table B.7.)
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 88AP: Find the total number of collisions between molecules in 1.00 s in 1.00 L of nitrogen gas at...
Related questions
Question
two parts to a question

Transcribed Image Text:!
Required information
For divers going to great depths, the composition of the air in the tank must be modified. The ideal composition is to
have approximately the same number of O2 molecules per unit volume as in surface air (to avoid oxygen poisoning),
and to use helium instead of nitrogen for the remainder of the gas (to avoid nitrogen narcosis, which results from
nitrogen dissolving in the bloodstream). Of the molecules in dry surface air, 78.0% are N2, 21.0% are 02, and 1.00% are
Ar. (Assume that the density of seawater is 1025 kg/m³ and the temperature is 20.0°C.) Density of dry air at 20.0°C is
1.20 kg/m3. Molar mass of N2 is 14.007 g/mol, 02 is 15.999 g/mol, and Ar is 39.948 g/mol. (See Table B.7.)
For a diver going to a depth of 153 m, what percentage of the gas molecules in the tank should be 02?
| %
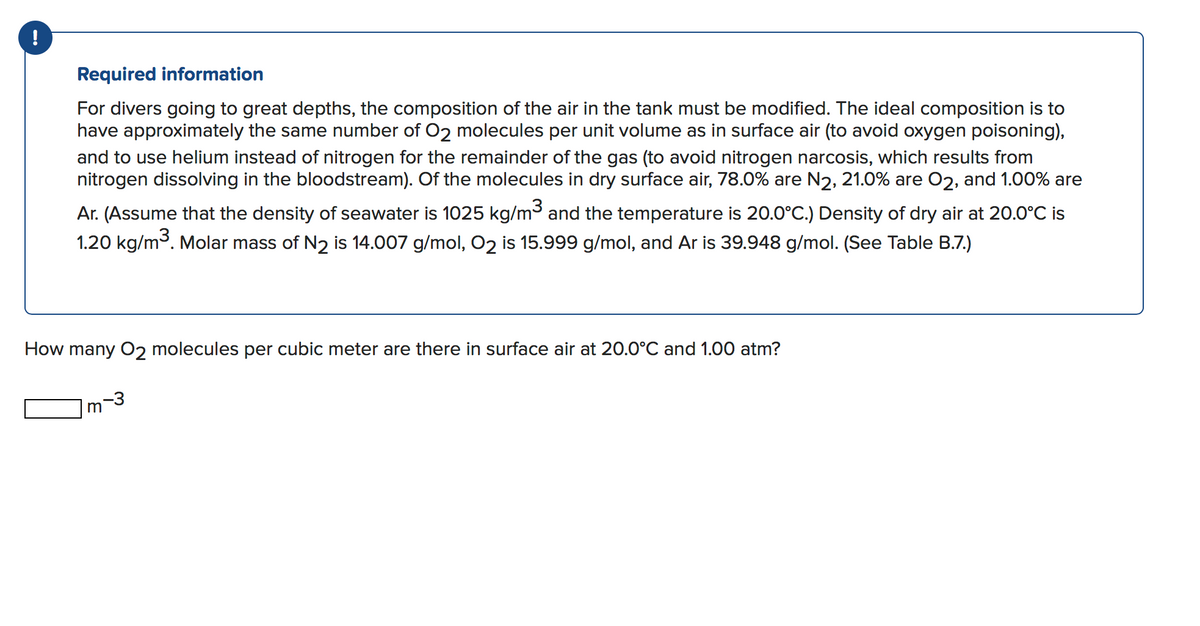
Transcribed Image Text:Required information
For divers going to great depths, the composition of the air in the tank must be modified. The ideal composition is to
have approximately the same number of O2 molecules per unit volume as in surface air (to avoid oxygen poisoning),
and to use helium instead of nitrogen for the remainder of the gas (to avoid nitrogen narcosis, which results from
nitrogen dissolving in the bloodstream). Of the molecules in dry surface air, 78.0% are N2, 21.0% are O2, and 1.00% are
Ar. (Assume that the density of seawater is 1025 kg/m³ and the temperature is 20.0°C.) Density of dry air at 20.0°C is
1.20 kg/m3. Molar mass of N2 is 14.007 g/mol, O2 is 15.999 g/mol, and Ar is 39.948 g/mol. (See Table B.7.)
How many 02 molecules per cubic meter are there in surface air at 20.0°C and 1.00 atm?
m
-3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University