For each calculation, you will be required to show detailed work steps. Make sure the number of each question is clear according to what was in the introductions and procedure document. Meaning, make sure that your use of the template doesn't mess up the numbering! Post lab questions 1) Find the percent by mass of NaCl in solution Use Equation editor here to show all your work! You can copy and paste these when you need more lines % by mass NaCl Put your answer here with units! 2) Find the molality of NaCl in solution Use Equation editor here to show all your work! You can copy and paste these when you need more lines molality of NaCl Put your answer here with units! 3) Find the density of solution Use Equation editor here to show all your work! You can copy and paste these when you need more lines density Put your answer here with units! 4) Find the molarity of NaCl in solution Use Equation editor here to show all your work! You can copy and paste these when you need more lines molarity Put your answer here with units!
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
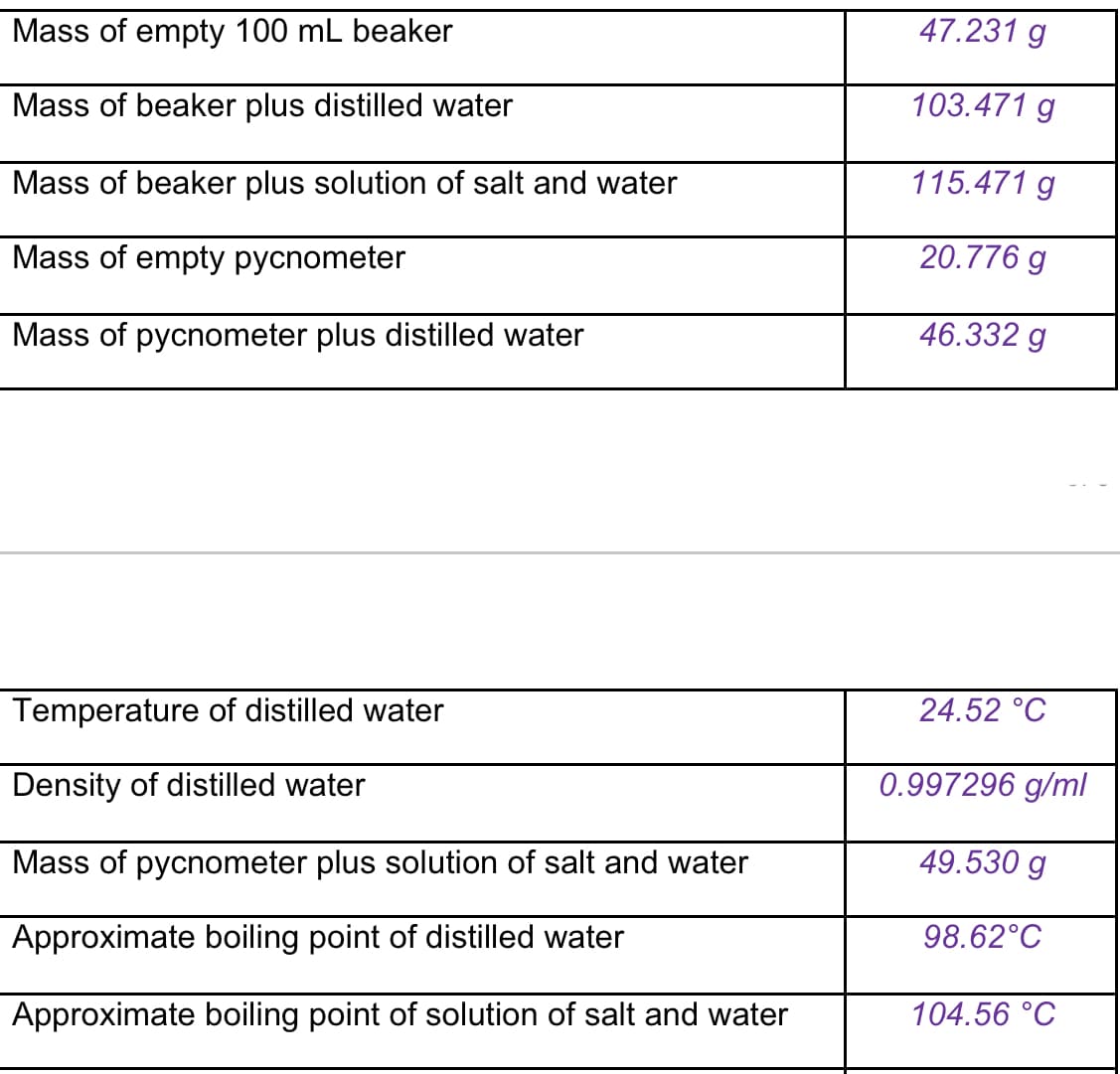
Hello, I need help on these questions if you can answer all of them? I gave you a data table and questions you can go ahead with. Some clarification is that for question 3 it's asking to find the the density of NaCI. And I also used 12 grams of sodium chloride.


Step by step
Solved in 3 steps with 3 images




