For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or redu highlighted atom is being... reaction neither oxidized reduced oxidized nor reduced FeO(s)+CO(g) → Fe(s)+CO,(9) NH3(aq)+2 O2(9) HNO3(aq)+H,O(1) 4 KI(aq)+2 Cu Cl,(aq) → 2 CuI(s)+I2(aq)+4 KCl(aq) 4 HF (g)+ SiO2(s) → SiF,(9)+2 H,O(g)
For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or redu highlighted atom is being... reaction neither oxidized reduced oxidized nor reduced FeO(s)+CO(g) → Fe(s)+CO,(9) NH3(aq)+2 O2(9) HNO3(aq)+H,O(1) 4 KI(aq)+2 Cu Cl,(aq) → 2 CuI(s)+I2(aq)+4 KCl(aq) 4 HF (g)+ SiO2(s) → SiF,(9)+2 H,O(g)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter22: The Chemistry Of The Transistion Elements
Section22.2: Metallurgy
Problem 1RC
Related questions
Question
100%
Recognising reduction and oxidation.
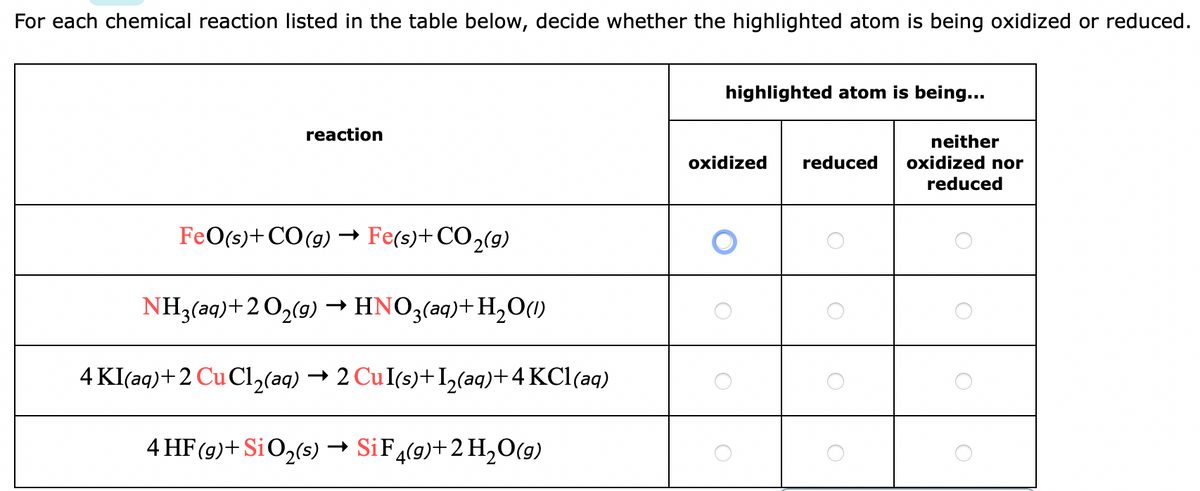
Transcribed Image Text:For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced.
highlighted atom is being...
reaction
neither
oxidized nor
oxidized
reduced
reduced
FeO(s)+CO(g) → Fe(s)+CO,(g)
NH3(aq)+2 O,(9) →HNO3(aq)+H,O(1)
4 KI(aq)+2 Cu Cl,(aq) → 2 CuI(s)+I,(aq)+4 KCl(aq)
4 HF(g)+ SiO2(s) → SiF,(9)+2 H20(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning