For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. ds Does solubility change with pH? highest solubility compound pH = 5 pH = 4 pH = 2 yes CACO, O no O yes CuBr O no O yes Ni(OH), O no oo o o o
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. ds Does solubility change with pH? highest solubility compound pH = 5 pH = 4 pH = 2 yes CACO, O no O yes CuBr O no O yes Ni(OH), O no oo o o o
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 82CP
Related questions
Question
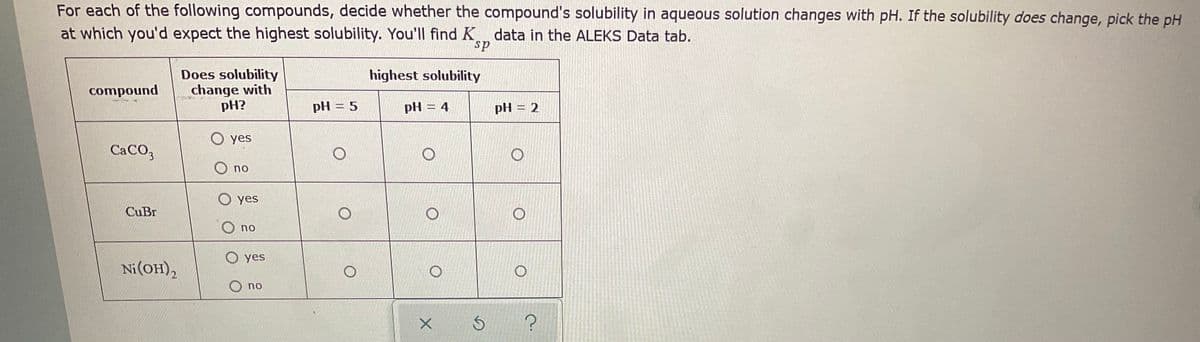
Transcribed Image Text:For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH
at which you'd expect the highest solubility. You'lIl find K, data in the ALEKS Data tab.
sp
Does solubility
change with
pH?
highest solubility
compound
pH = 5
pH = 4
pH = 2
O yes
CaCO,
O no
O yes
CuBr
O no
O yes
Ni(OH),
O no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax